Atropine sulfate ocular inserts and preparation method thereof
A tropine sulfate and ophthalmic technology, applied in the field of medicine, can solve problems such as low bioavailability, poor drug stability, and difficulty in eye drug delivery systems, and achieve the effects of solving eye discomfort, film transparency, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The present invention also provides the preparation method of the atropine sulfate eye film described in the above-mentioned technical scheme, comprises the following steps:
[0032] Mixing the film former, water and plasticizer in turn to obtain a slurry;
[0033] Atropine sulfate is added to the slurry, followed by film forming, drying and sterilization to obtain the atropine sulfate ophthalmic film.
[0034] In the present invention, the water is preferably water for injection. In the present invention, there is no special limitation on the dosage of the water for injection, as long as the complete mixing of the film-forming agent and the plasticizer is sufficient.
[0035] In the present invention, the film forming and drying are preferably carried out in a coater, the wet film coating thickness of the coater is preferably 100±20 μm, the coating speed is preferably 10 to 20 rpm, and the drying temperature is preferably 60 ±5°C.
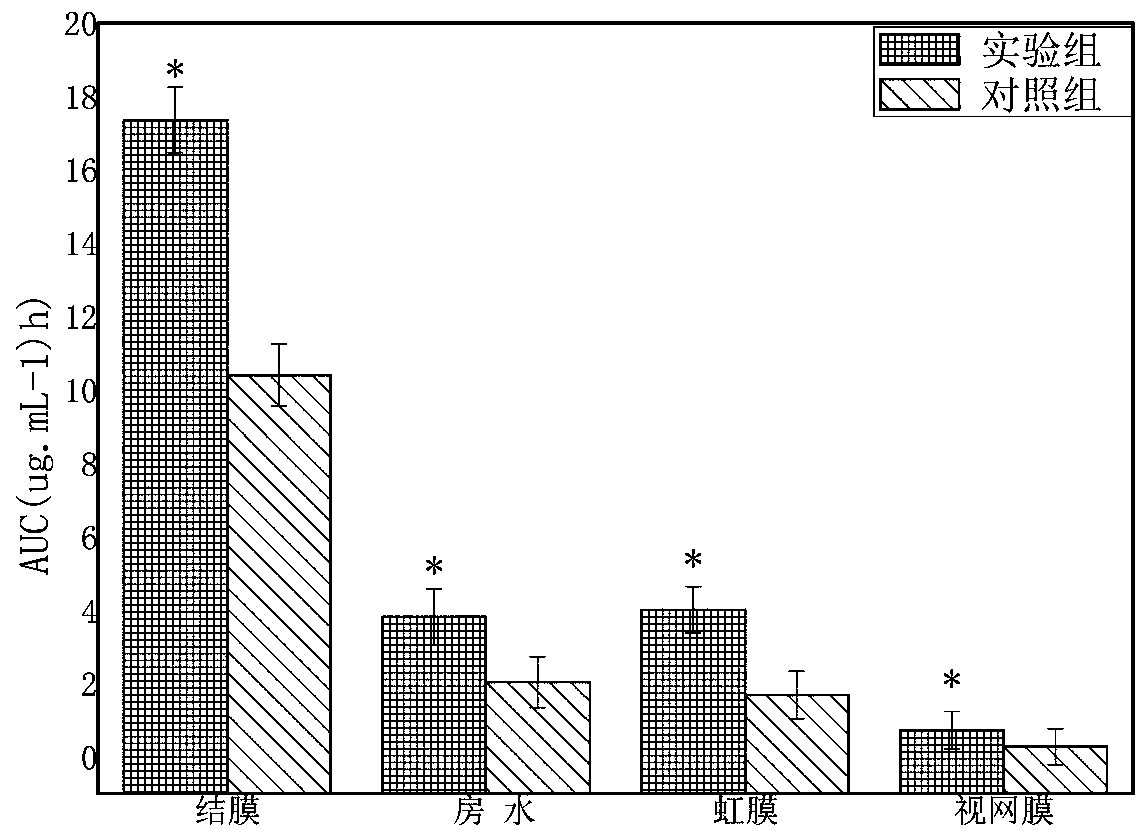
[0036] The present invention has ...
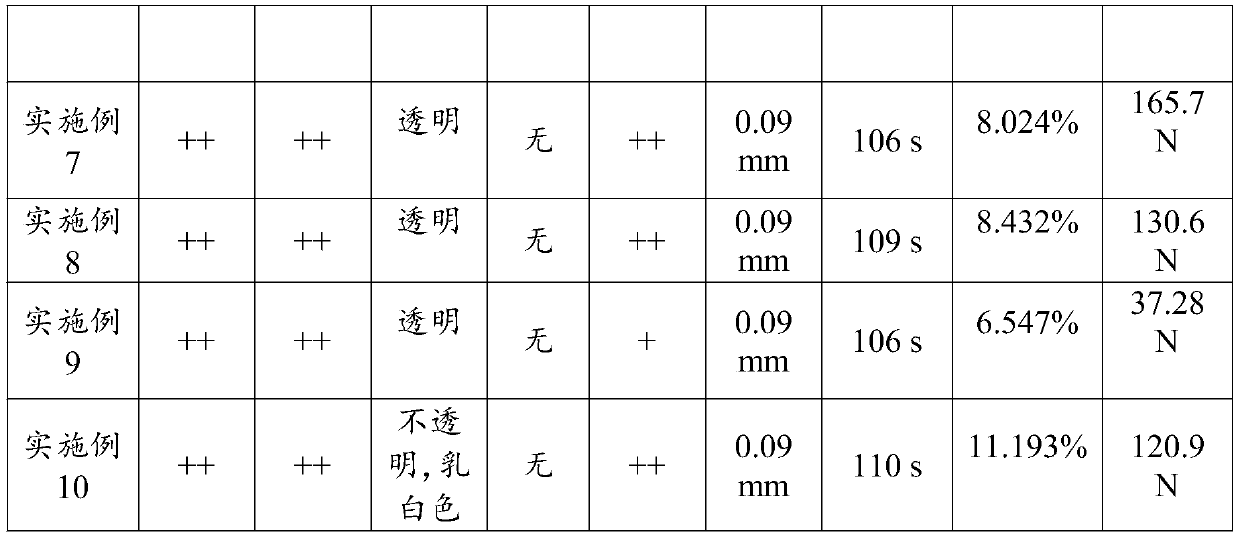
Embodiment 1
[0042] Atropine sulfate 31.5mg (8μg per square centimeter)
[0043] Hydroxypropyl methylcellulose E536g (9mg per square centimeter)
[0044] Glycerin 18g (4.5mg per square centimeter)
[0045] Water for injection 900mL
[0046] Preparation method: Accurately weigh the prescribed amount of hydroxypropyl methylcellulose E536g, add the prescribed amount of water for injection, fully stir, and dissolve for later use; then slowly add the prescribed amount of glycerin and atropine sulfate to the slurry, and stir at 1000rpm for 20 minutes; Get the slurry. Turn on the coater, install the backing material, adjust the coating thickness so that the thickness of the film is about 100±20μm, set the coating speed to 10rpm, evenly coat the slurry on the backing to form a film, dry at 60±5°C, and dry After that, the film is complete, with uniform appearance and good flexibility.
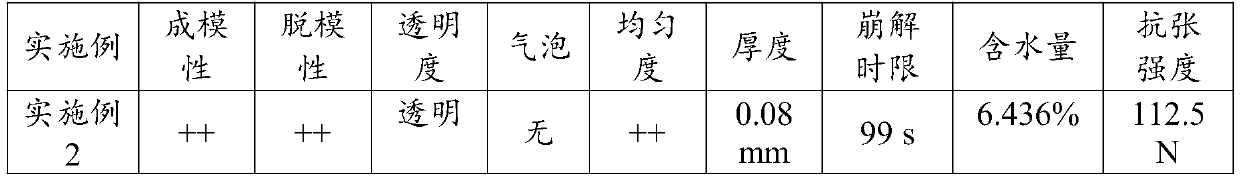
Embodiment 2
[0048]Atropine sulfate 31.5mg (8μg per square centimeter)
[0049] Hydroxypropyl methylcellulose E1536g (9mg per square centimeter)
[0050] Glycerin 18g (4.5mg per square centimeter)
[0051] Water for injection 900mL
[0052] Preparation method: Accurately weigh the prescribed amount of hydroxypropyl methylcellulose E1536g, add the prescribed amount of water for injection, fully stir, and dissolve for later use; then slowly add the prescribed amount of glycerin and atropine sulfate to the slurry, and stir at 1000rpm for 60min; Get the slurry. Turn on the coater, install the backing material, adjust the coating thickness so that the thickness of the film is about 100±20μm, set the coating speed to 20rpm, evenly coat the slurry on the backing to form a film, dry at 60±5°C, and dry Finally, the film is complete, the appearance of the film is uniform, and the flexibility is better than that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com