Long-acting pharmaceutical preparation combination of leuprolide acetate and use method of pharmaceutical preparation combination
A technology for leuprolide acetate and pharmaceutical preparations, applied in directions such as drug combination, drug delivery, and pharmaceutical formulations, can solve the problems such as the inability to increase the drug load, reduce the sudden release, etc., so as to solve the problem that the drug load cannot be increased and reduce the sudden release, cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
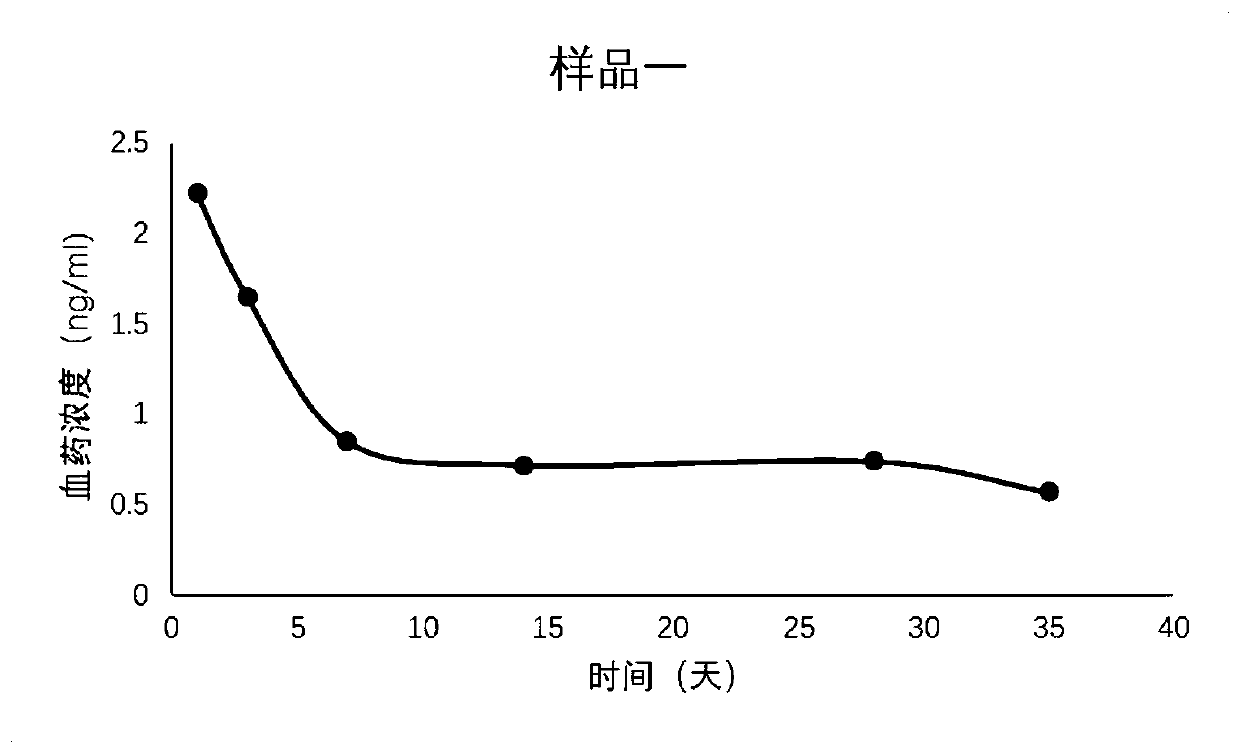
[0041] A combination of leuprolide acetate long-acting pharmaceutical preparations, comprising leuprolide acetate, biodegradable polymer microspheres and solvents stored independently; the biodegradable polymer microspheres are microspheres formed from a mixture of PLGA and PCL ball.
[0042] The specific preparation method of the biodegradable polymer microspheres is:
[0043] (1) Dissolve 55 g of PLGA (lactide: glycolide = 50 / 50, self-made, MW: 41000) and 5 g of PCL (self-made, MW: 10000) in 200 ml of dichloromethane, and filter and sterilize to obtain organic Mutually;
[0044] (2) Using a pressure of 1500mpa, the organic phase is squeezed through the membrane tube, the membrane hole is 15 μm, squeezed into the continuous water phase, the flow rate of the water phase is 600ml / min, the microspheres are extruded, the emulsion is volatilized and dried, and the microspheres are solidified and formed. Under GMP conditions, it is aseptically dispensed into vials, 60 mg / bottle; ...
Embodiment 2
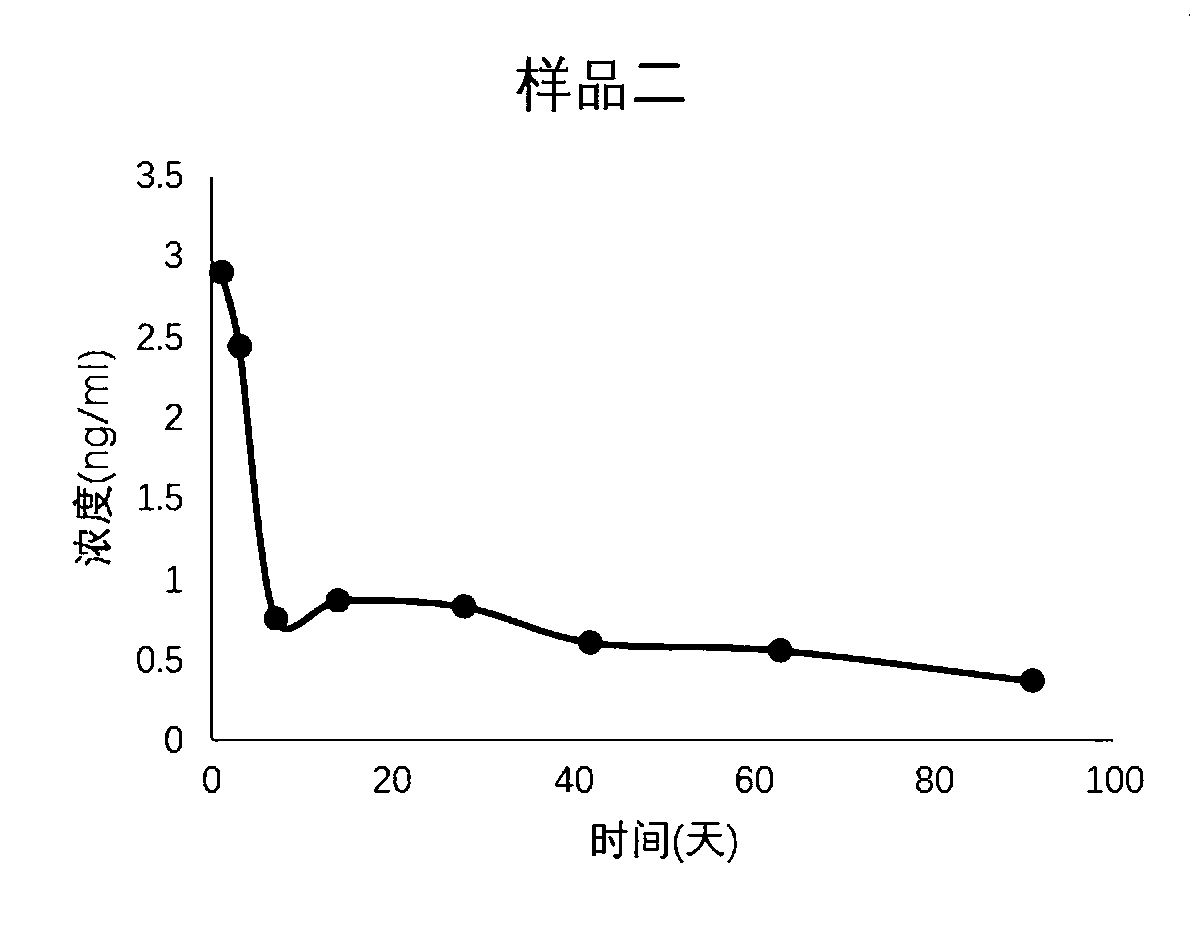
[0048] A combination of leuprolide acetate long-acting pharmaceutical preparations, comprising leuprolide acetate, biodegradable polymer microspheres and solvents stored independently; the biodegradable polymer microspheres are microspheres formed from a mixture of PLGA and PCL ball.
[0049] The specific preparation method of the biodegradable polymer microspheres is:
[0050] (1) Dissolve 50 g of PLGA (lactide: glycolide = 75 / 25, self-made, MW: 32000) and 10 g of PCL (self-made, MW: 10000) in 200 mL of dichloromethane, and filter and sterilize to obtain organic Mutually;
[0051] (2) Using a pressure of 500mpa, the organic phase is squeezed through the membrane tube, the membrane hole is 50 μm, squeezed into the continuous water phase, the flow rate of the water phase is 1000ml / min, the microspheres are extruded, the emulsion is volatilized and dried, and the microspheres are solidified and formed. Under GMP conditions, it is aseptically dispensed into vials, 110 mg / bottle...
Embodiment 3
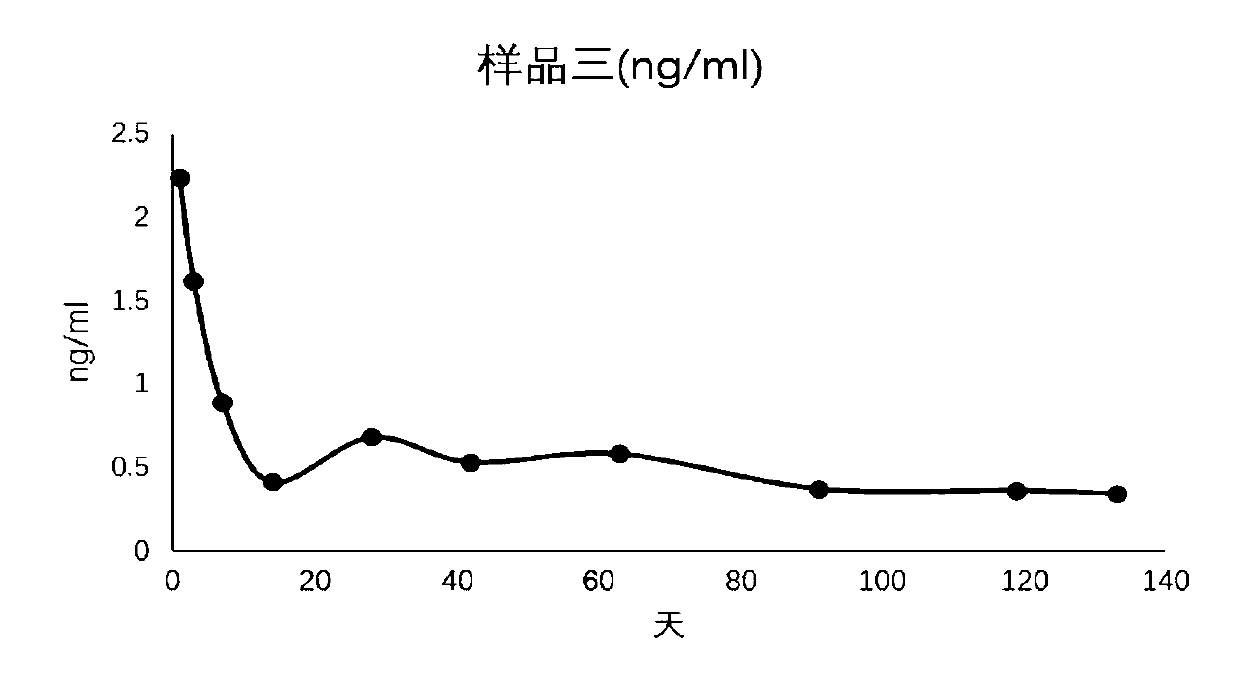
[0055] A combination of leuprolide acetate long-acting pharmaceutical preparations, comprising leuprolide acetate, biodegradable polymer microspheres and solvents stored independently; the biodegradable polymer microspheres are microspheres formed from a mixture of PLGA and PCL ball.
[0056] The specific preparation method of the biodegradable polymer microspheres is:
[0057] (1) Dissolve 50 g of PLGA (lactide: glycolide = 85 / 15, self-made, MW: 41000) and 10 g of PCL (self-made, MW: 10000) in 200 mL of dichloromethane, and filter and sterilize to obtain organic Mutually;
[0058] (2) Using a pressure of 2000mpa, the organic phase is squeezed through the membrane tube, the membrane hole is 30 μm, squeezed into the continuous water phase, the flow rate of the water phase is 5000ml / min, the microspheres are extruded, the emulsion is volatilized and dried, and the microspheres are solidified and formed. Under GMP conditions, it is aseptically dispensed into vials, 130 mg / bottl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com