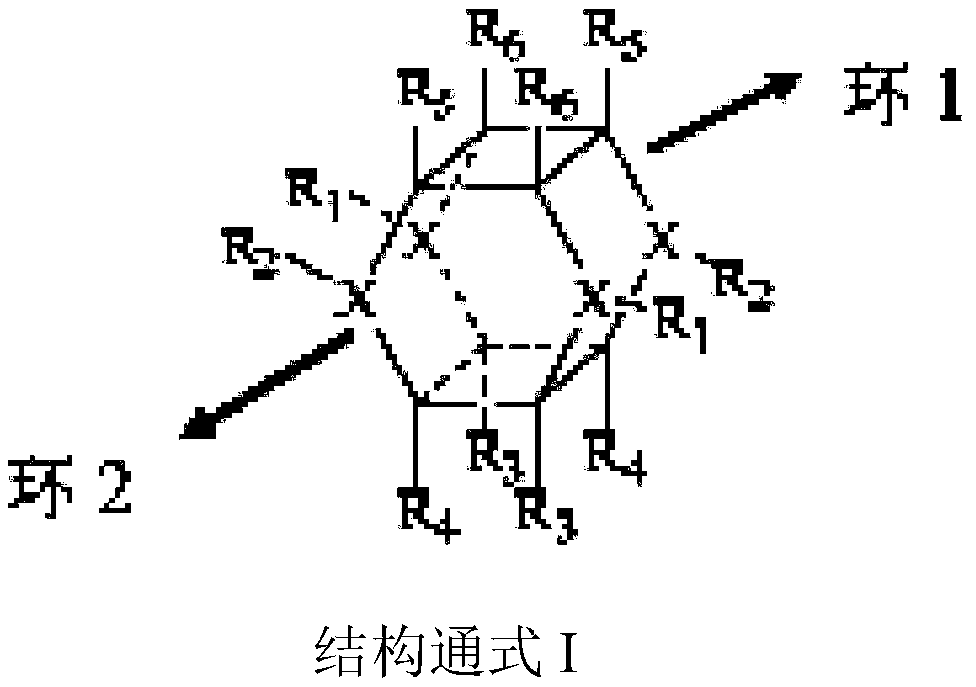
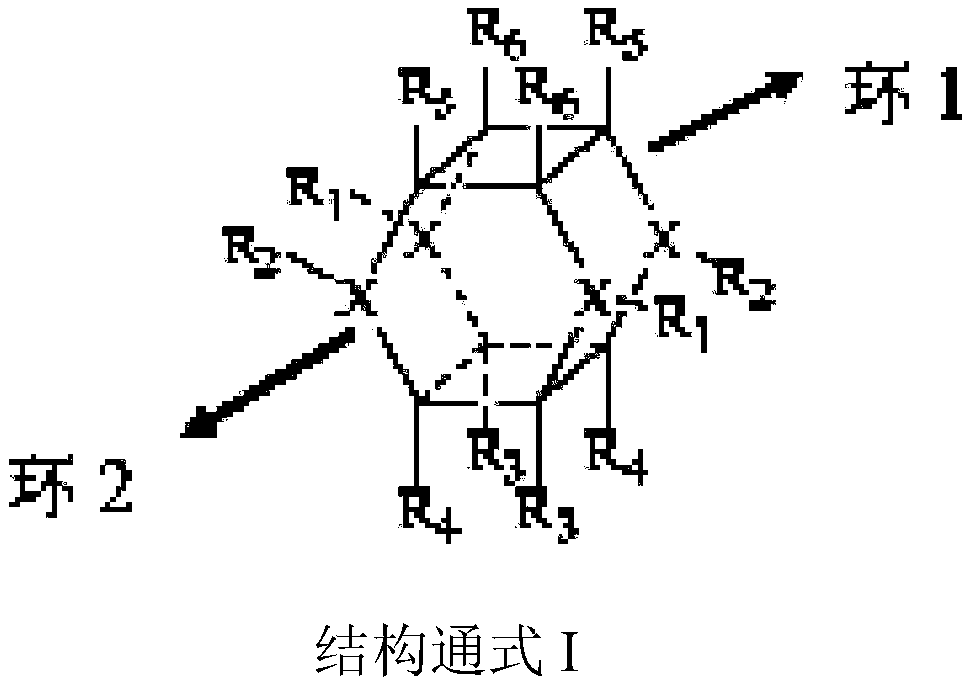
Visible light preparation method for azatraasterane compounds
A technology of azatetrastartane and visible light, which is applied in organic chemistry and other fields, and can solve the problems of many by-products, low wavelength selectivity, and short service life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
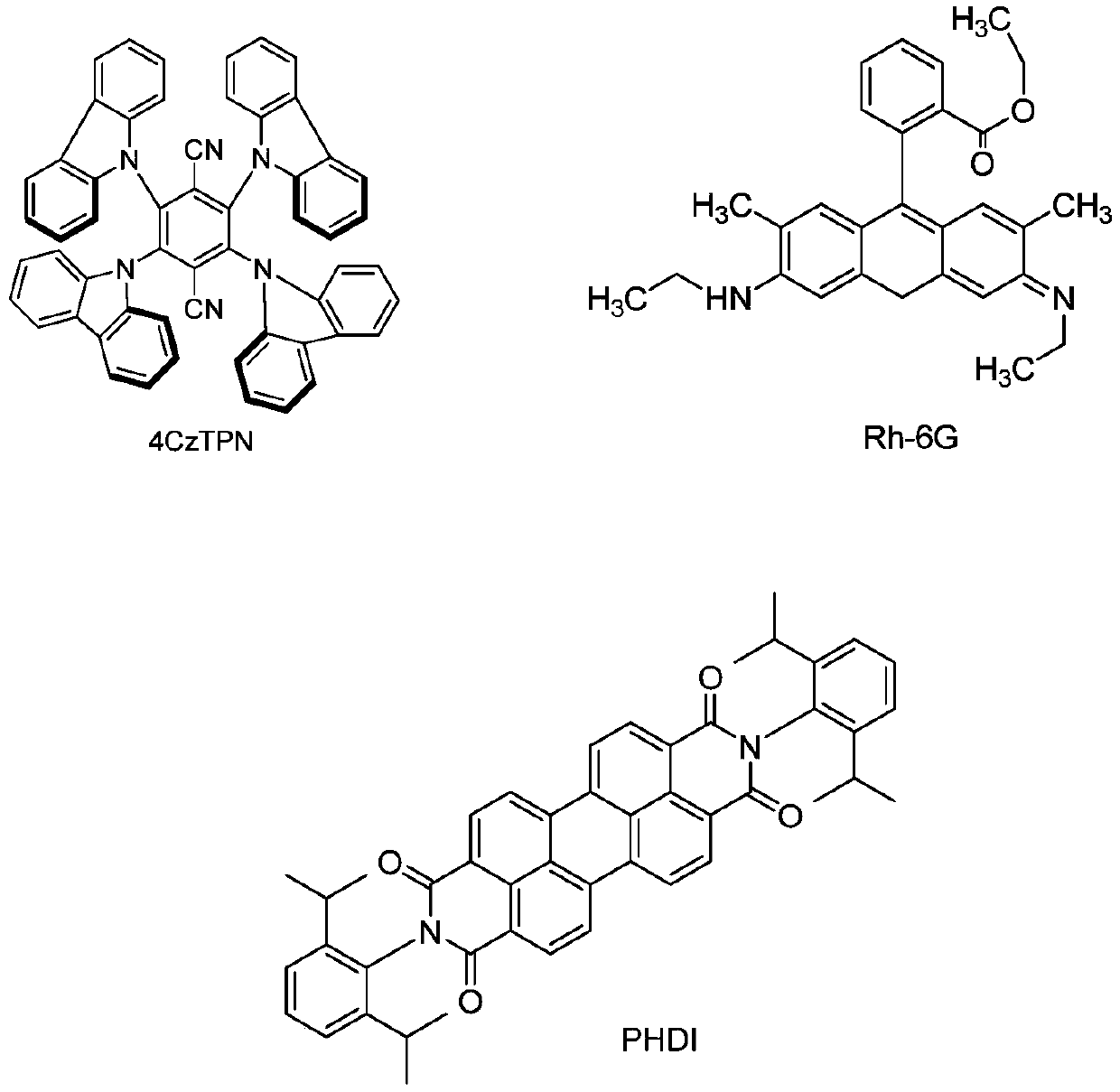
[0021] Weigh 2.91g (10mmol) of ethyl 1,4-diphenyl-1,4-dihydropyridine-3-carboxylate and 0.23g (0.5mmol) of visible light catalyst Flavin into a round bottom flask, dissolve in 20ml tetrahydrofuran In a mixed solvent with methanol (V:V=1:1), under nitrogen protection, the resulting reaction solution was irradiated at room temperature under a 50W white light energy-saving lamp light source, and the light source was 20cm away from the reactant. The reaction was tracked by TLC. After the reaction was complete, the target product 3,6,9,12-tetraphenyl-7,11-dicarboxylic acid ethyl ester-3,9-diazatetrapentane could be obtained by direct filtration.
[0022]
[0023] 1 H NMR (400MHz, CDCl 3 )δ H (ppm):7.34-6.88(m,20H),5.33(d,2H),4.95(d,2H),4.20-4.04(m,4H),3.73(d,2H),3.53(td,2H), 1.10(t,6H); 13 C NMR (100MHz, CDCl 3 )δ C (ppm):174.56,150.58,140.65,129.35,128.97,128.05,126.18,119.44,116.85,60.94,55.87,53.87,53.69,40.76,38.62,13.97.HRMS(ESI + )m / z[M+H] + C 40 h 39 N 2 o 4 T...
Embodiment 2
[0025] Weigh 2.91g (10mmol) of ethyl 1,4-diphenyl-1,4-dihydropyridine-3-carboxylate and 0.55g (0.7mmol) of visible light catalyst 4CzTPN into a round bottom flask, dissolve in 20ml THF In a mixed solvent with methanol (V:V=1:1), under nitrogen protection, the resulting reaction solution was irradiated under a 10W blue LED light source at room temperature, and the light source was 20 cm away from the reactant. The reaction was tracked by TLC. After the reaction was complete, the target product 3,6,9,12-tetraphenyl-7,11-dicarboxylic acid ethyl ester-3,6-diazatetrapentane could be obtained by direct filtration.
[0026]
[0027] 1 H NMR (400MHz, CDCl 3 )δ H (ppm):7.31-6.88(m,20H),5.30(d,2H),4.59-4.54(d,2H),4.35(d,2H),4.21-4.08(m,4H),3.37(d,2H ), 1.10(t, 6H); 13C NMR (100MHz, CDCl 3 )δ C (ppm): 174.55, 149.74, 141.27, 129.06, 128.77, 128.15, 125.76, 119.90, 116.31, 61.23, 55.60, 50.79, 49.65, 44.10, 39.53, 14.02. HRMS (ESI + )m / z[M+H] + C 40 h 39 N 2 o 4 The theoreti...
Embodiment 3
[0029] Weigh 3.24g (10mmol) of ethyl 1-(4-bromophenyl)-1,4-dihydropyridine-3,5-dicarboxylate and 0.32g (0.5mmol) of visible light catalyst Eosin Y into a round bottom flask , dissolved in 20ml of ethanol solvent, under nitrogen protection, and placed the resulting reaction solution at room temperature under a 50W white light energy-saving lamp light source, and the light source was 20cm away from the reactant. TLC followed the reaction. After the reaction was complete, directly filter to obtain the target product 3,9-(4-bromophenyl)-1,5,7,11-ethyl tetracarboxylate-3,9-diazatetrastar alkyl.
[0030]
[0031] 1 H NMR (400MHz, CDCl 3 )δ H (ppm):7.01-7.24(m,12H),6.62(d,4H),4.83(m,4H),4.16(d,6H),2.21(s,2H),1.96(d,4H),1.21( d,4H); 13 C NMR (100MHz, CDCl 3 )δ C (ppm): 177.9, 148.6, 132.5, 116.5, 116.2, 71.8, 61.9, 47.6, 38.9, 14.1. HRMS (ESI + )m / z[M+H] + C 34 h 36 Br 2 N 2 o 8 The theoretical calculation value is 760.4852, and the detection value is 760.4856.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com