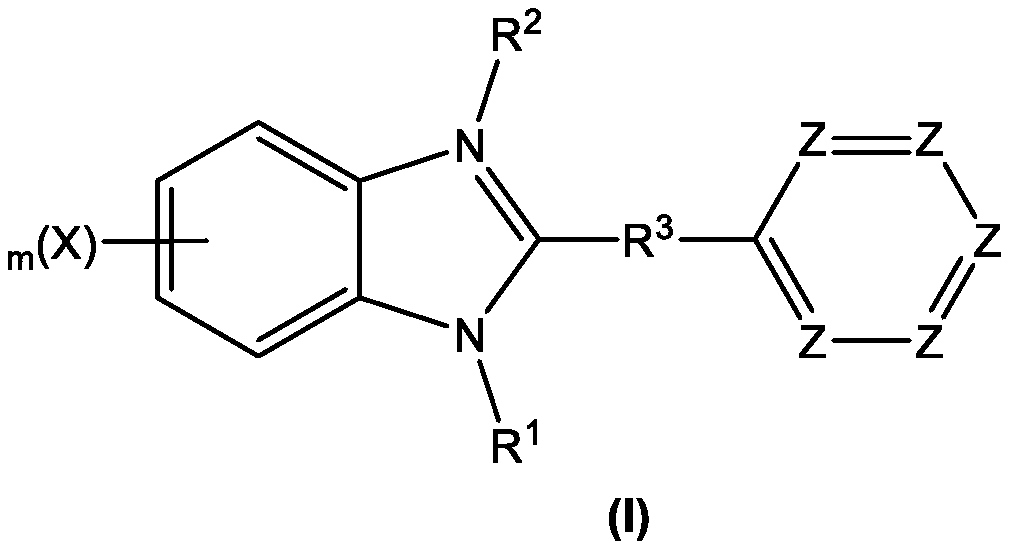
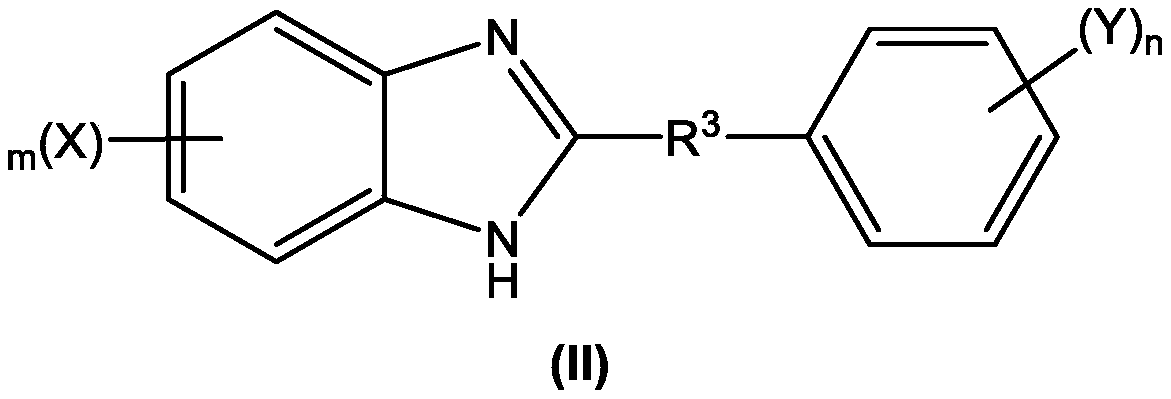
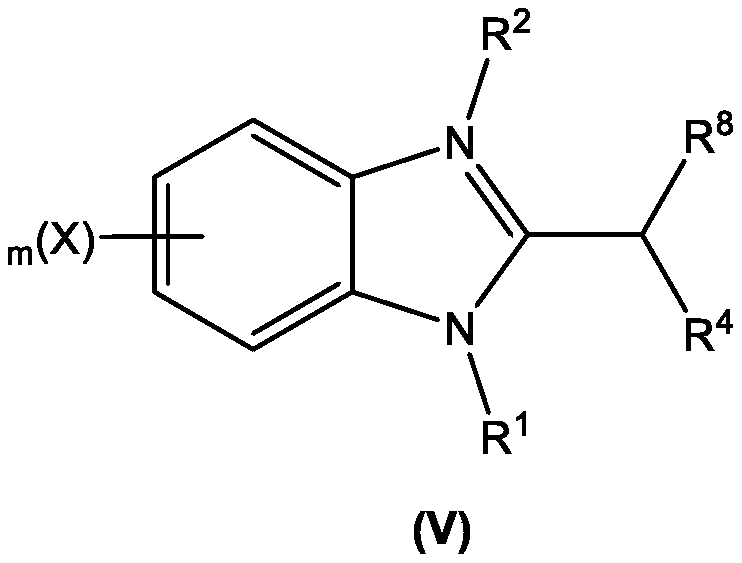
A novel one-pot homogeneous process for the large scale manufacture of 2-substituted benzimidazoles
A kind of unsubstituted, C4-C6 technology, applied in the homogeneous process of benzimidazole and its field as corrosion inhibitor for example, can solve the problem of processing and separation step yield loss, large capital investment, prolong reaction and processing time etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] Several condensation reactions between 1,2-phenylenediamine (OPD) and DL-mandelic acid yielded (1H-benzo[d]imidazol-2-yl)(phenyl)methanol at about 30 to about 35 wt% The active material is carried out at 100-110° C. for about 6 to about 8 hours. The effects of various additives (such as high-temperature stable catalysts and co-solvents) on the homogeneity of the reaction were studied, and are listed in Table 1.
[0163] Table 1: Various additives evaluated
[0164]
[0165] *HEG-Cl=hexaethyleneguanidine chloride
[0166] **Ambient temperature
Embodiment 2
[0168] Several experiments were performed using about 5 wt% sulfolane and about 3 wt% acetic acid in the work-up step. The results of HPLC analysis are shown in Table 2. The results clearly show that the reduction in residual OPD concentration is less than about 0.1 wt%, and the resulting material complies with regulations. Active substance refers to the weight percent concentration of OPD and DL-mandelic acid at the beginning of the reaction.
[0169] Charge methanesulfonic acid, sulfolane, and water into a round bottom flask equipped with a magnetic stirrer, reflux condenser, and temperature probe. DL-mandelic acid (1 equivalent) and 1,2-phenylenediamine (1 equivalent) were added thereto, and the contents of the flask were refluxed at about 100-110° C. for about 6-8 hours. After the reaction was complete, glacial acetic acid (3 wt%) was added and reflux was maintained for about another 1-3 hours. After work-up, additional water was added to adjust the active substance to ...
Embodiment 3
[0172] Example 3: Synthesis of 1H-benzo [d] imidazol-2-yl) (phenyl) methanol
[0173]
[0174] Charge methanesulfonic acid, sulfolane, and water into a flask equipped with a magnetic stirrer, reflux condenser, and temperature probe. To this was added o-phenylenediamine (about 12.96 g, 1 equiv) and DL-mandelic acid (about 19.15, 1.05 equiv), and the contents of the flask were refluxed at about 110°C. After about 8 hours of reflux, about 3 g of acetic acid was added and the reflux was maintained for about 3 hours to obtain the title compound in about 97% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com