A crystal form of urat1 inhibitor and its preparation method
A technology of crystal form and map, applied in the field of crystal form and preparation of URAT1 inhibitor, can solve the problem that the additional effect is not very significant and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1: the preparation of formula (I) compound
[0070]
[0071] synthetic route:
[0072]
[0073] Step 1: Synthesis of Compound 2
[0074] Add 4.5 L of dimethyl sulfoxide to a three-neck flask (10 L), add potassium tert-butoxide (836.66 g, 7.46 mol, 2 eq) under stirring, and continue stirring for 10 minutes until the solution is clear, and then cool to The internal temperature of the reaction solution is 20-25°C. Add compound 1 (500.05g, 3.73mol, 1eq) dimethyl sulfoxide (500mL) solution dropwise to the above solution, after dropping, stir and react for 30 minutes, then add carbon disulfide (283.86g, 3.73mol, 1eq ), dropwise, continued stirring reaction for 30 minutes. Ethyl bromoacetate (1250 g, 7.46 mol, 2 eq) was added dropwise thereto, and the reaction was continued to stir for 2 hours after the drop was completed. Finally, potassium carbonate (515.52 g, 7.46 mol, 1 eq) was added, and the temperature was raised to 65° C. to continue stirring for 8 ...
Embodiment 2
[0098] Embodiment 2: Preparation of the A crystal form of the compound of formula (I)
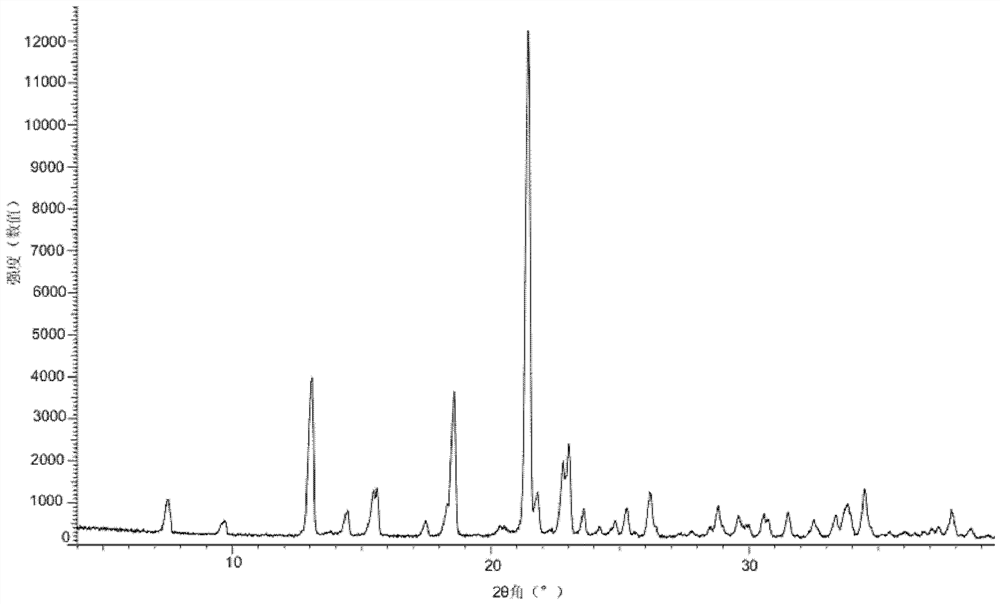
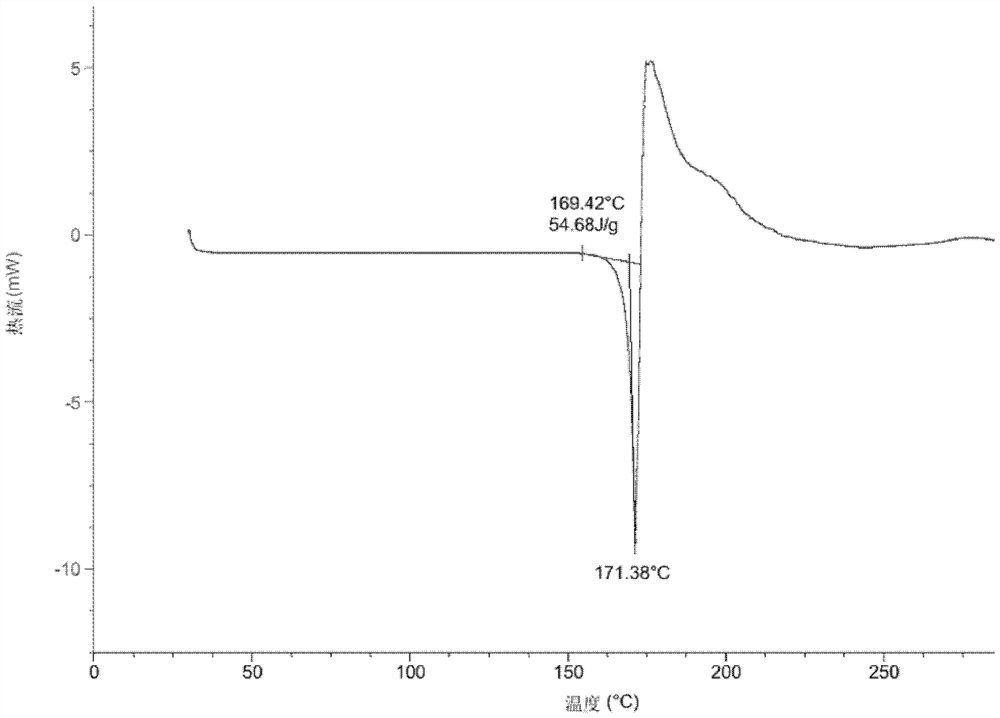
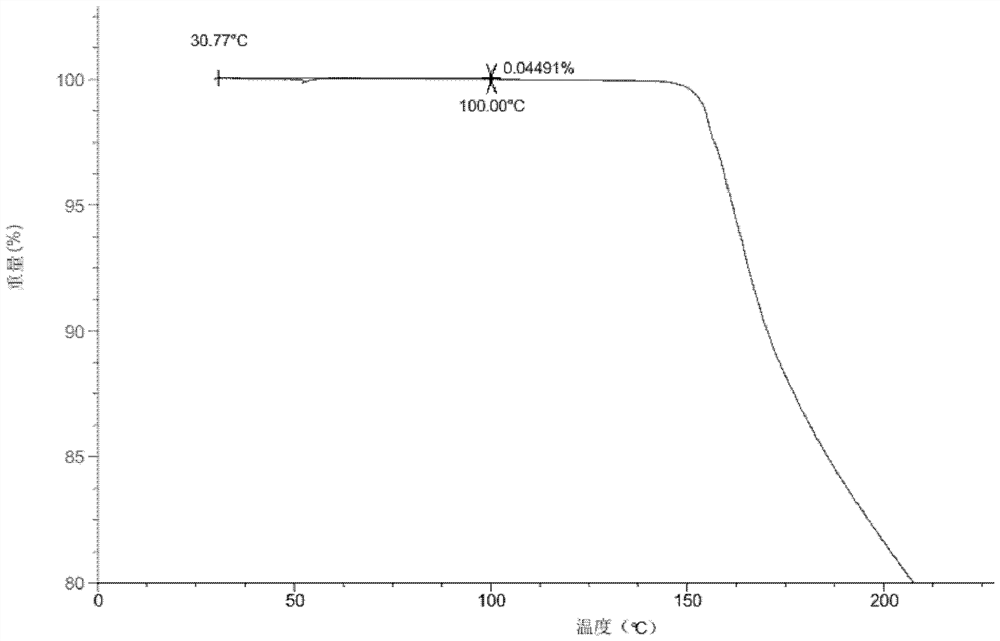
[0099] Add the compound of formula (I) (50mg) into a glass bottle, add methanol (0.4mL) respectively, and stir to form a suspension or solution. The above suspension sample was placed in a constant temperature mixer (40° C.), shaken at 40° C. for 60 hours, and then centrifuged to collect the sample. The above dissolved samples were volatilized at room temperature and then centrifuged to collect the samples. The above sample was dried overnight in a vacuum drying oven (40° C.), and its crystal form was detected by XRPD, and the crystal form of the final product obtained was Form A of the compound of formula (I).
[0100]Add the compound of formula (I) (50mg) into a glass bottle, add ethyl acetate (0.4mL) respectively, and stir to form a suspension or solution. The above suspension sample was placed in a constant temperature mixer (40° C.), shaken at 40° C. for 60 hours, and then centrifuge...
Embodiment 3
[0101] Embodiment 3: Preparation of the B crystal form of the compound of formula (I)
[0102] Add the compound of formula (I) (50 mg) into a glass bottle, add tetrahydrofuran (0.4 mL), and stir until it dissolves. The above dissolved samples were volatilized at room temperature and then centrifuged to collect the samples. The collected samples were dried overnight in a vacuum drying oven (40° C.), and the crystal form state was detected by XRPD. The crystal form of the final product obtained was Form B of the compound of formula (I).
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com