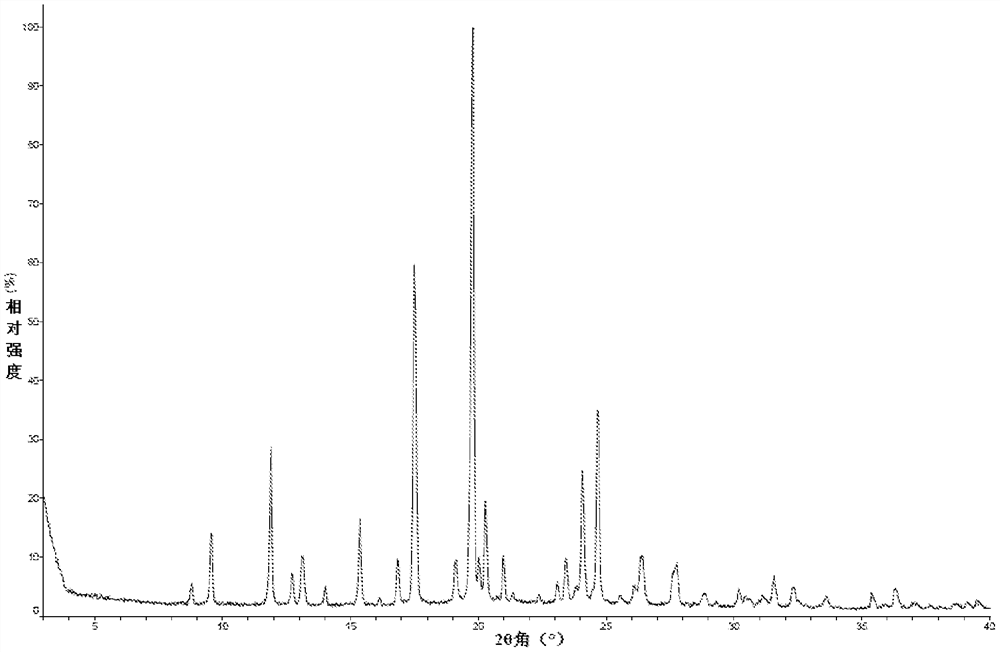
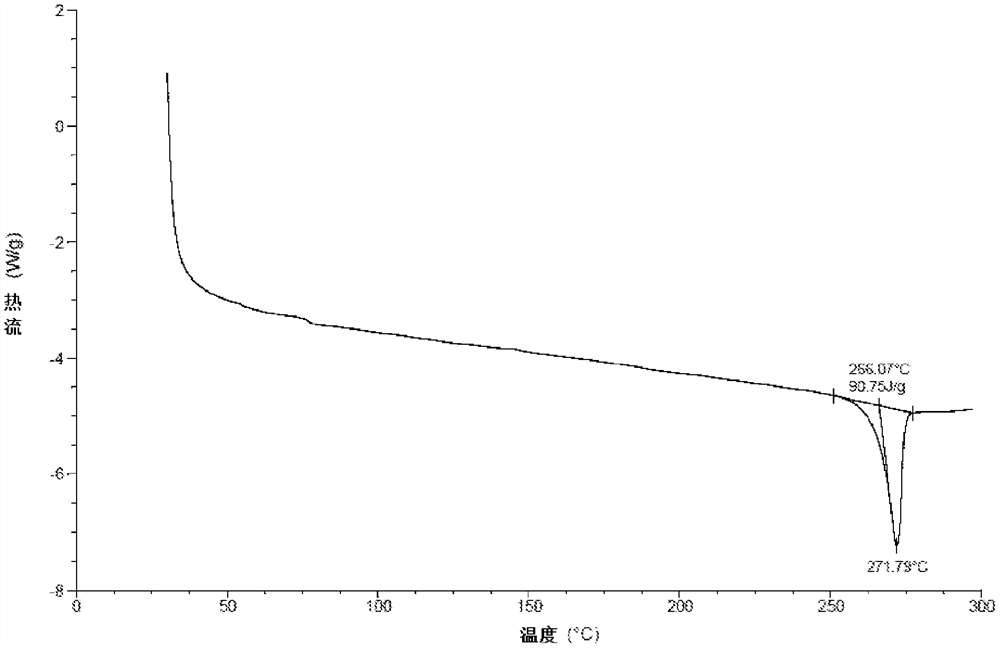
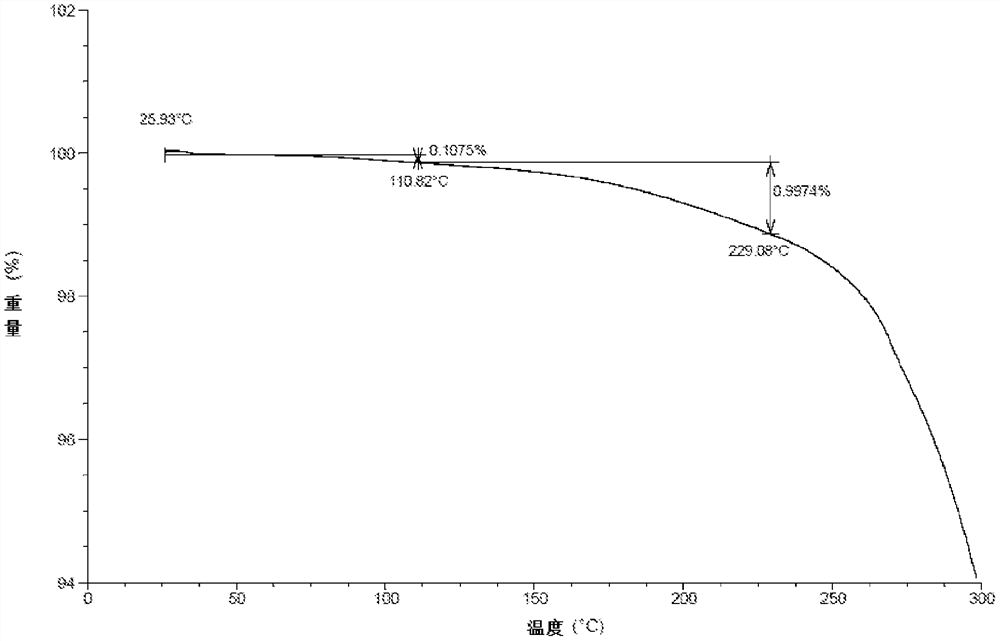
A kind of crystal form, salt form and preparation method of TGF-βRI inhibitor
一种晶型、化合物的技术,应用在制备治疗癌症药物中的应用领域,能够解决信号转导通路异常等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] The preparation of embodiment 1 formula (I) compound
[0101]
[0102] Preparation of Intermediates 1-6:
[0103]
[0104] Step A: Ethyl acetate (291.41 mL, 2.98 mol) was dissolved in toluene (750.00 mL), then sodium ethoxide (135.06 g, 1.98 mol) was added in portions at room temperature, and the reaction mixture was stirred at room temperature for 1 hour. 1-1 (150.00 g, 992.33 mmol) was added to the above reaction solution at 25°C, then heated to 95°C and stirred for 15 hours. The reaction mixture was cooled to about 30°C, adjusted to pH 7 with acetic acid, diluted with water (500 mL), and extracted with ethyl acetate (500 mL). The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column (eluent: petroleum ether / ethyl acetate v / v=50 / 1) to obtain 1-2.
[0105]Step B: Dissolve 1-2 (120.00 g, 579.07 mmol) in pyridine (300 mL), then add 1-aminopyrrolidin-2-one p-toluenesu...
Embodiment 2
[0115] The preparation of embodiment 2 formula (II) compound
[0116]
[0117] Add 115 mg of the compound of formula (I) into an 8 ml glass bottle, add 4 ml of tetrahydrofuran, sonicate to help dissolve, and form a suspension; then slowly add 1.05 equivalents of p-toluenesulfonic acid monohydrate. The above suspended sample was placed on a magnetic stirrer (40° C.) and stirred for 16 hours. The sample solution was centrifuged, and the solid was taken out and dried in a vacuum oven at 35° C. for 16 hours to obtain the compound of formula (II). 1 H NMR (400MHz, CD 3 OD) δ8.61(s, 1H), 8.14(t, J=8.0Hz, 1H), 8.05(d, J=15.6Hz, 1H), 7.90(d, J=8.8Hz, 1H), 7.70(dd , J=8.4, 15.6Hz, 4H), 7.54(d, J=15.6Hz, 1H), 7.39(d, J=8.0Hz, 1H), 7.20(d, J=7.6Hz, 2H), 4.42(m , 2H), 3.05-2.87 (m, 2H), 2.82 (s, 3H), 2.81-2.74 (m, 2H), 2.35 (s, 3H).
Embodiment 3
[0118] The preparation of embodiment 3 formula (IV) compound
[0119]
[0120] Add 115 mg of the compound of formula (I) into an 8 ml glass bottle, add 4 ml of tetrahydrofuran, sonicate to help dissolve, and form a suspension; then slowly add 1.05 equivalents of hydrochloric acid. The above suspended sample was placed on a magnetic stirrer (40° C.) and stirred for 16 hours. The sample solution was centrifuged, and the solid was taken out and dried in a vacuum oven at 35°C for 16 hours. Add appropriate amount of acetone to the obtained solid to form a suspension and stir at 40°C, centrifuge to discard the supernatant, and drain the solid sample with an oil pump at room temperature to obtain the compound of formula (IV).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com