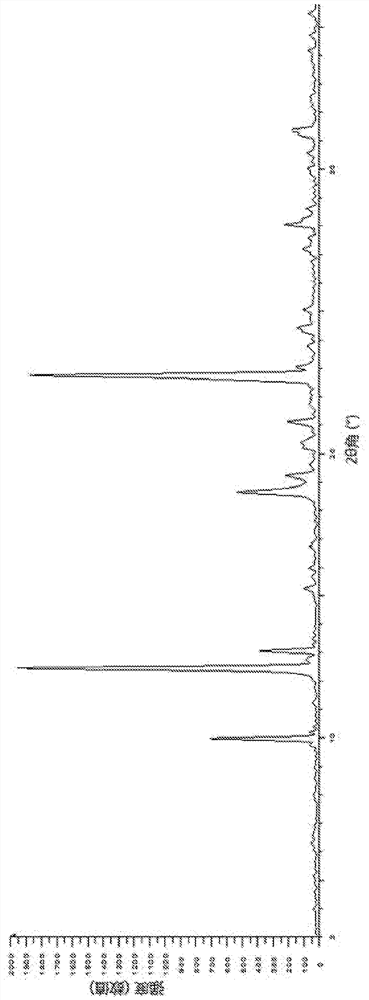
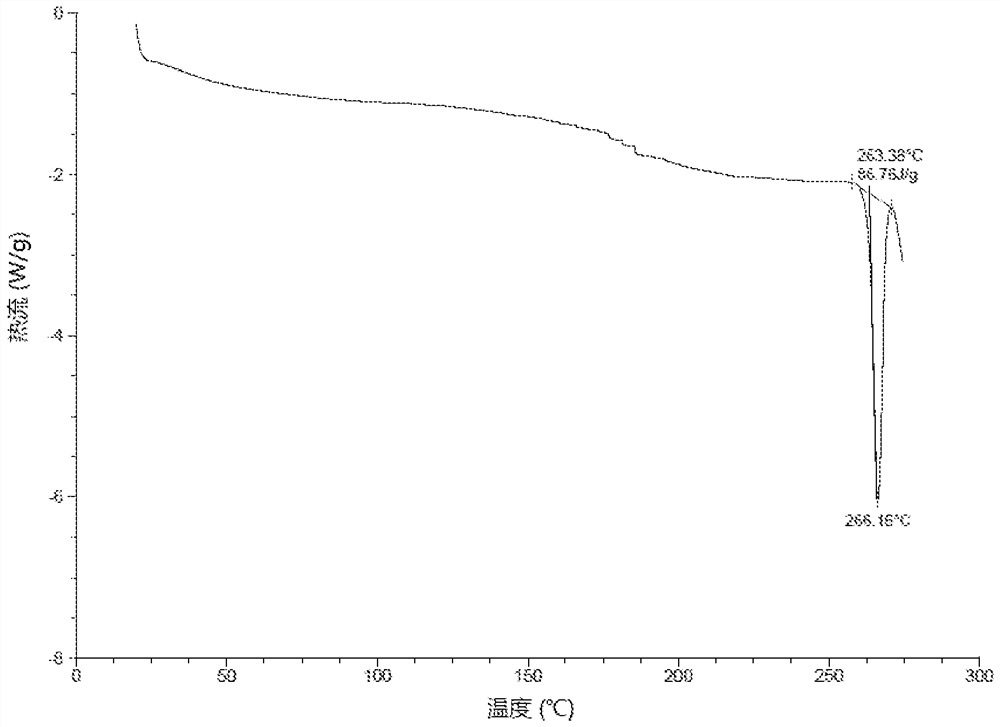
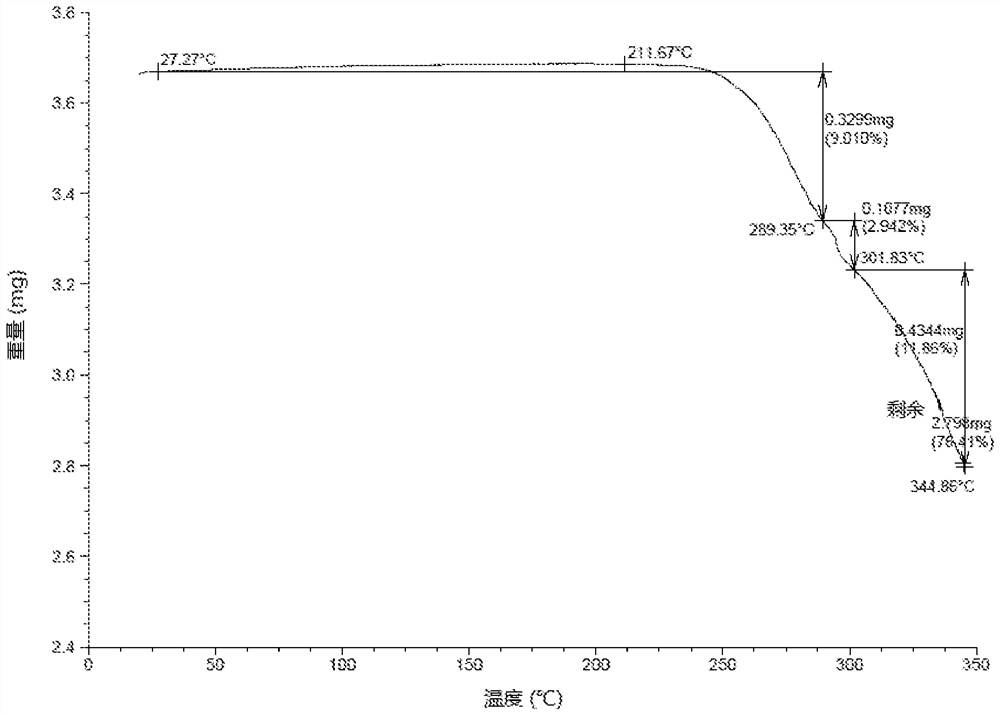
Salt and crystal forms of pyridopyridone derivatives
A technology of crystal form and hydrochloride, applied in the field of salt form of novel pyridopyridone derivatives, can solve the problems of large dose, obvious side effects in the gastrointestinal tract, difficult to penetrate the blood-brain barrier, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0108] Preparation of the crystal form of the compound of formula (I-2)
[0109] At 18°C, THF (2.8 L) and the compound of formula (I) (288.25 g, 528.09 mmol, 1.0 eq) were successively added into the reaction kettle, and heated to 40°C, the system was a little cloudy. The mixture was filtered while it was hot, and the filtrate was re-transferred to the reactor, and concentrated hydrochloric acid (39.60 ml, 0.9 equivalents) was slowly added dropwise to the reactor, then THF (280 ml) was added, and stirred at 40-45°C for 16 Hour. The reaction mixture was filtered, the filter cake was washed with THF (280 mL), and the filter cake was dried in a vacuum oven at 50° C. for 4 hours to obtain a crude product.
[0110] At room temperature of 12°C, the above crude product (257.16 g), EtOH (2.9 L) and water (145 ml) were successively added to the reaction kettle, and the reaction mixture was heated to 80°C (vigorous reflux), and the reaction system became clear. Heating was stopped and ...
Embodiment 1
[0112] Embodiment 1: the hygroscopicity research of the crystalline form of compound of formula (I-2)
[0113] Experimental conditions:
[0114] Instrument model: SMSDVS Advantage dynamic vapor adsorption instrument
[0115] Test conditions: Take a sample (10-15 mg) and place it in a DVS sample tray for testing.
[0116] DVS parameters:
[0117] Temperature: 25°C
[0118] Balance: dm / dt=0.01% / min (shortest: 10min, longest: 180min)
[0119] Drying: 120min at 0% RH
[0120] RH(%) test rung: 10%
[0121] RH (%) test step range: 0%-90%-0%
[0122] See Table 1-2 for the evaluation criteria of hygroscopicity.
[0123] Table 1-2
[0124] Hygroscopicity Classification Moisture absorption weight gain*(ΔW%) deliquescence Absorb enough water to form a liquid Very hygroscopic ΔW%≥15% Hygroscopic 15%>ΔW%≥2% slightly hygroscopic 2%>ΔW%≥0.2% No or almost no hygroscopicity ΔW%
[0125] Note: *Weight gain under moisture absorption at ...
Embodiment 2
[0128] Embodiment 2: the crystal form research of formula (I-2) compound
[0129] Weigh about 50 mg of the crystal form of the compound of formula (I) into different 1.5 mL glass vials, and add an appropriate amount of solvent or solvent mixture (see Table 1-3 below) to form a suspension or solution. After adding magnets, the above samples were placed on a magnetic heating stirrer (40° C.) for 2 days of testing.
[0130] Table 1-3
[0131] Numbering solvent crystal form 1 ethanol The crystal form of the compound of formula (I-2) 2 Acetonitrile The crystal form of the compound of formula (I-2) 3 acetone The crystal form of the compound of formula (I-2) 4 ethyl acetate The crystal form of the compound of formula (I-2) 5 Tetrahydrofuran The crystal form of the compound of formula (I-2)
[0132] It can be seen from the above table that under the solvent conditions of ethanol, acetonitrile, acetone, ethyl acetate and tetrah...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com