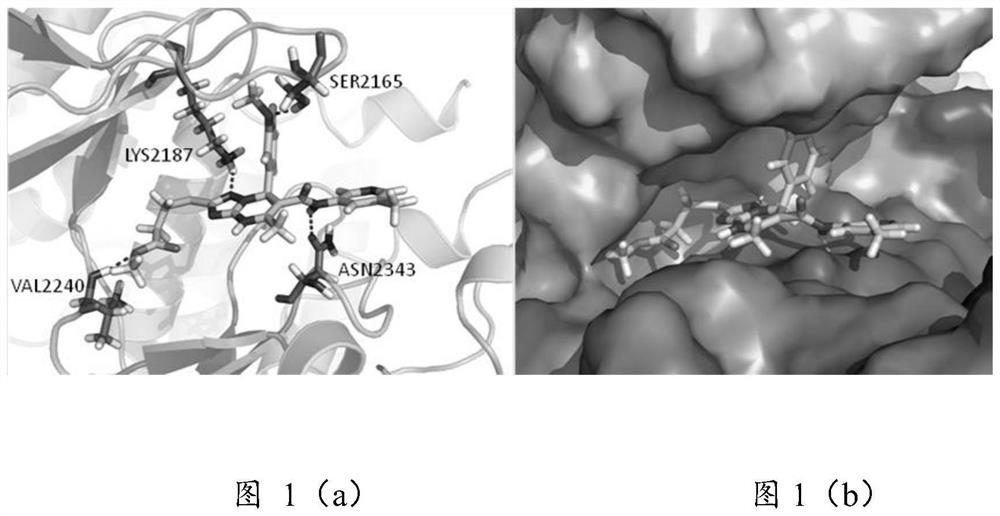
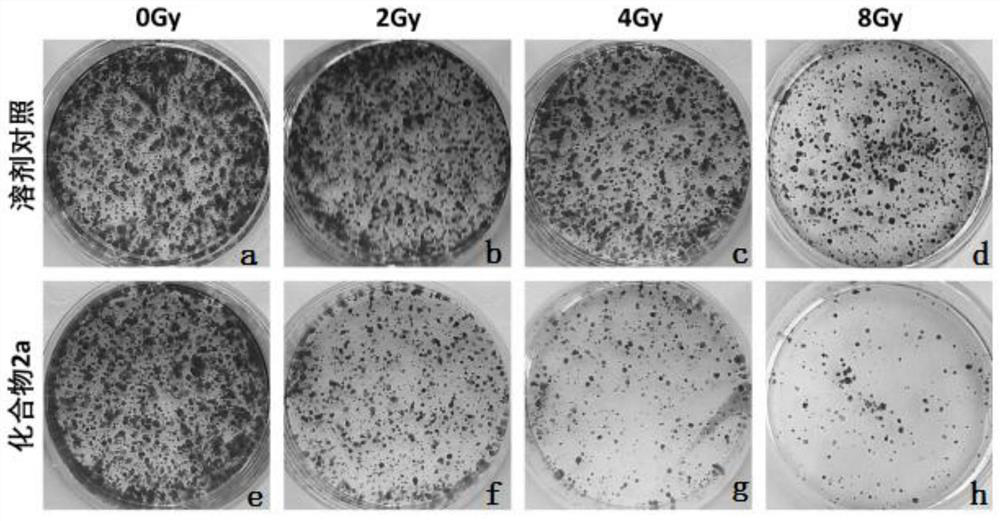
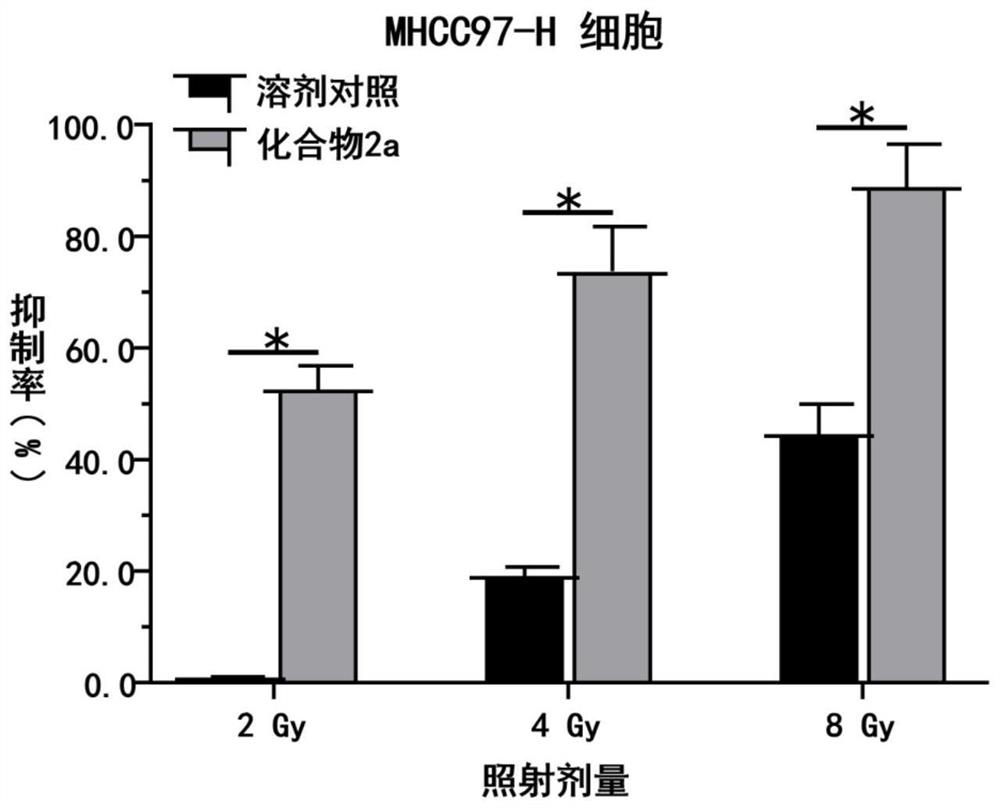
A kind of triazolopyrimidine compound and its preparation method and application
An azolopyrimidine and compound technology, which is applied in the field of triazolopyrimidine compounds and their preparation, and achieves the effects of good mTOR inhibitory activity, good drug prospect and good anticancer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075]
[0076] Methyl 3-((7-(3-methoxyphenyl)-5-methyl-6-((4-methylpyridin-3-yl)carbamoyl)-4,7-dihydro-[1 ,2,4]Synthesis of triazol[1,5-a]pyrimidin-2-yl)thio)propionate (2a)
[0077] Step 1: Preparation of methyl 3-((5-amino-1H-1,2,4-triazol-3-yl)thio)propionate (intermediate 1)
[0078] Methyl 3-bromopropionate (14.36g, 0.086mol) was dissolved in 40ml of absolute ethanol for later use. In a 500ml reaction vessel, NaOH (3.44g, 0.086mol) was dissolved in 200ml of purified water and stirred until completely dissolved. 3-Amino-5-thio-1,2,4-triazole (10.00 g, 0.086 mol) was then added and stirred. After 30 minutes, a mixture of methyl 3-bromopropionate and ethanol was added. React overnight at room temperature. After the reaction was complete, it was extracted with ethyl acetate, washed with saturated sodium chloride and washed with Na 2 SO 4 dry. Methyl 3-((5-amino-1H-1,2,4-triazol-3-yl)thio)propanoic acid (11.65 g, yield 67%) was obtained.
[0079] Step 2: Preparatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com