New Lorlatinib crystal form and preparation method thereof
A lorlatinib crystal form and lorlatinib technology, which are applied in organic chemistry methods, organic chemistry and other directions, can solve the problems of low product purity, lack of druggability, incomplete crystallisation and the like, and achieve a simple preparation process, Improves bioavailability in vivo and facilitates dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Preparation of lorlatinib crystal form LX-1
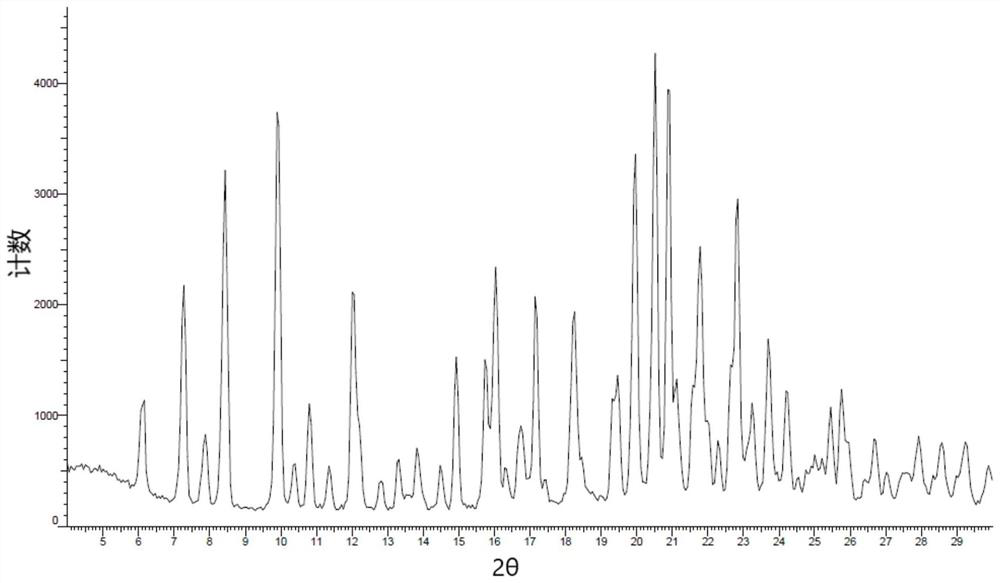
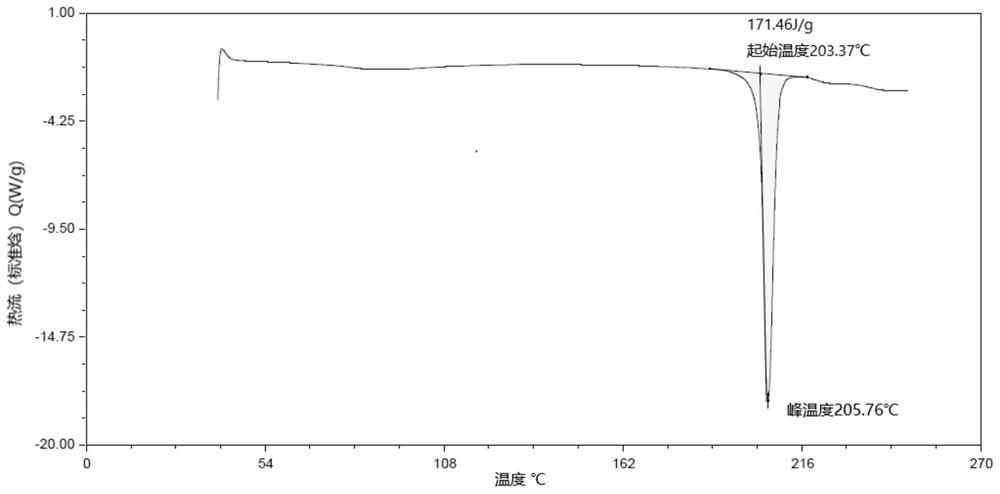
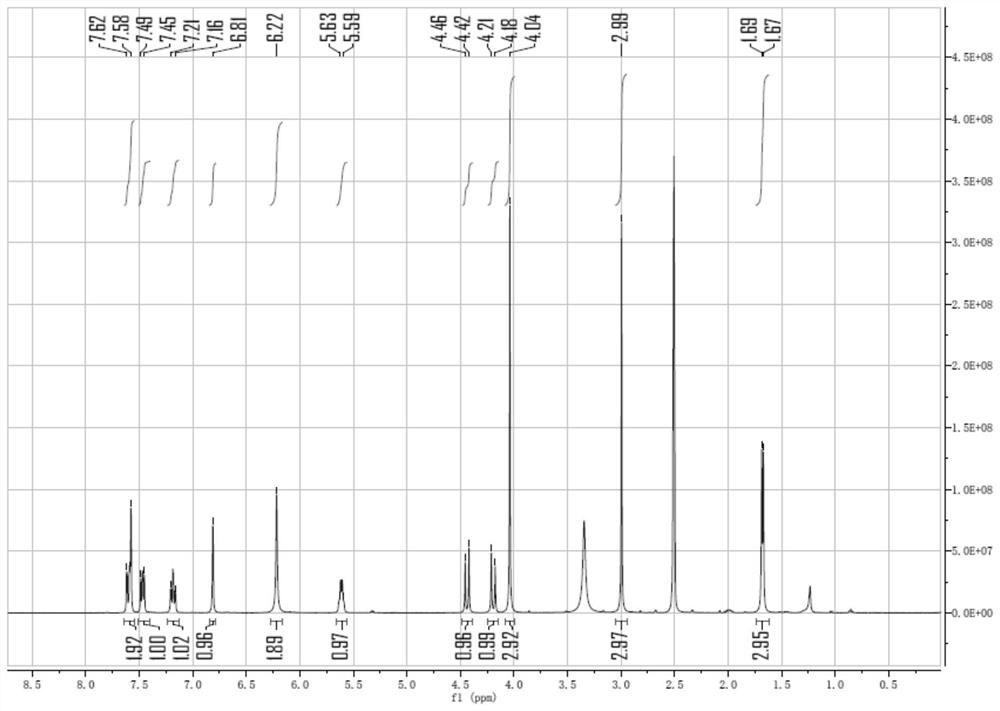
[0125] Dissolve 20 mg of lorlatinib (Form 24 or amorphous) in dimethyl sulfoxide at room temperature at a concentration of 200 mg / ml-250 mg / ml. In this example, lorlatinib (Form 24 or amorphous) is controlled The concentration is 200mg / ml, and the solid powder is obtained by rapid volatilization at room temperature and placed in a 60°C oven for 2 hours to obtain the crystal form LX-1. The XRD spectrum is shown in the appendix figure 1 , DSC spectrum see attached figure 2 , 1 See attached for HNMR spectrum image 3 .
Embodiment 2
[0127] Preparation of lorlatinib crystal form LX-1
[0128] Dissolve 20 mg of lorlatinib (Form 24 or amorphous) in dimethyl sulfoxide at 60°C with a concentration of 300 mg / ml-500 mg / ml. In this example, the control of lorlatinib (Form 24 or amorphous ) at a concentration of 350 mg / ml, dissolved and filtered, cooled at -20°C, and the obtained solid powder was placed at 80°C and continuously vacuumed for 0.5h, which was Lorlatinib crystal form LX-1, and its identification data With embodiment 1.
Embodiment 3
[0130] Preparation of lorlatinib crystal form LX-1
[0131] Dissolve 20 mg of lorlatinib (Form 24 or amorphous) in dimethyl sulfoxide at room temperature, and its concentration is 200 mg / ml-250 mg / ml. In this example, lorlatinib (Form 24 or amorphous ) at a concentration of 200 mg / ml, liquid-liquid diffusion and gas-liquid diffusion at room temperature in the antisolvent, wherein the antisolvent is 5 times that of the good solvent dimethyl sulfoxide, and the obtained solid powder is placed at 60 ° C for 1 hour to continuously vacuumize, which is Lorlatinib crystal form LX-1, wherein the anti-solvent used is isopropyl ether or methyl tert-butyl ether, and its identification data are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com