Il-4-fusion formulations for treatment of central nervous system (CNS) tumors
A central nervous system and glioma technology, applied in the field of IL-4 fusion preparations for the treatment of central nervous system (CNS) tumors, can solve problems such as no treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0356] Example 1: Treatment of recurrent or progressive glioblastoma with PRX 321
[0357] An open-label, nonrandomized, multicenter phase 2 study of PRX 321 in convection-enhanced delivery (CED) in adults with recurrent or progressive glioblastoma
[0358] Fundamental:
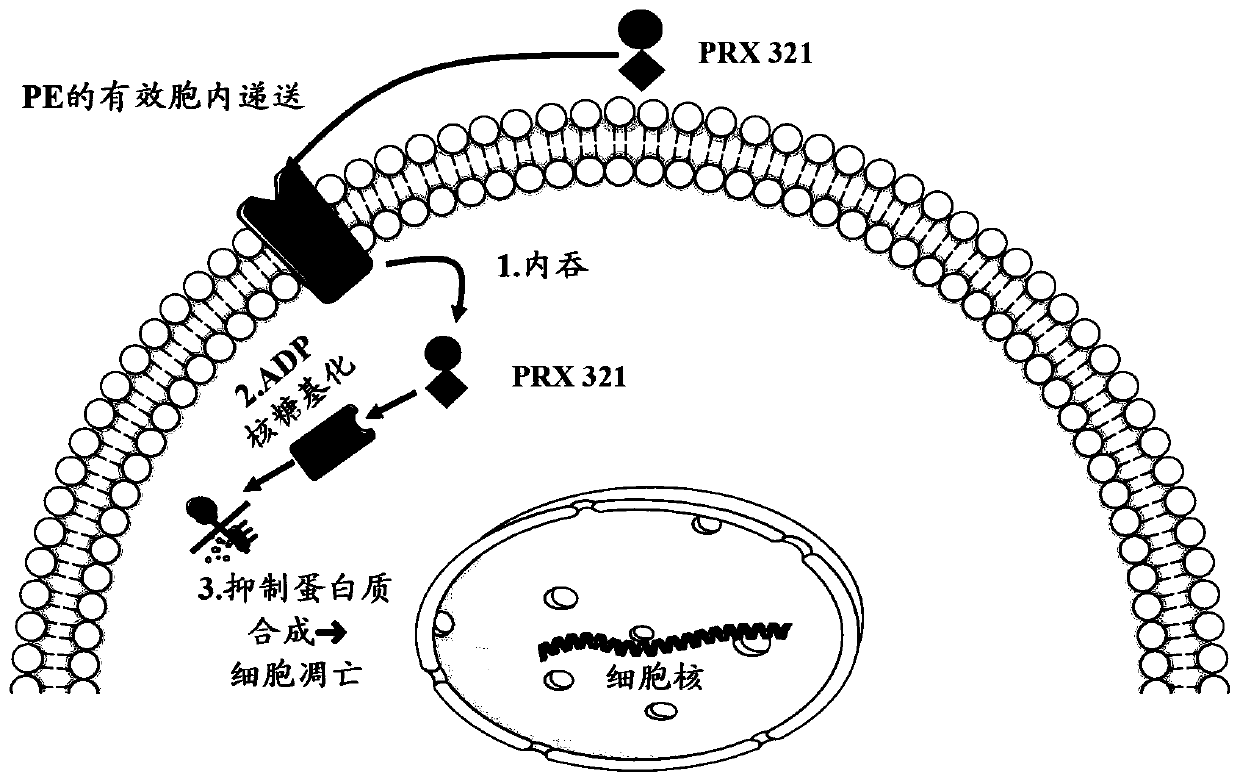
[0359] PRX 321 is a fusion toxin comprising genetically engineered circularly permuted interleukin-4 (cpIL-4) fused to a modified version of Pseudomonas aeruginosa exotoxin A (PE). PRX 321 binds to the IL-4 receptor (IL-4R) overexpressed by cancer cells and non-malignant immunosuppressive cells of the tumor microenvironment (TME) and delivers the potent cell-killing agent PE. A large percentage of glioblastoma (GB) and their TME express relatively high amounts of IL-4R, making IL-4R a relevant target for PRX 321. Intratumoral and peritumoral infusions minimized systemic exposure to fusion toxins, while image-guided CED technology enhanced active drug exposure across the target area. PRX 321 shares many p...
Embodiment 2
[1104] Example 2: PRX 321 formulation
[1105] introduction
[1106] This example provides more details on an open-label, single-arm, multicenter study of intratumoral administration of PRX321 in patients with recurrent or progressive glioblastoma (GB). Up to 52 subjects will receive a single intratumoral infusion of PRX 321 at a fixed concentration of 3 μg / mL via convection-enhanced delivery (CED), in an infusion volume of 60 mL, administered through up to 4 surgically placed catheters, as administered Detailed description in Example 1. The duration of the infusion is expected to be in the range of 24 to 36 hours, depending on the flow rate and number of convective catheters; however, if the infusion is required for completion, it can last up to 48 hours.
[1107] PRX 321 Medicines at Elliotts solution to produce a mixture with PRX 321 (3 μg / mL), 0.02% human serum albumin and gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA, ) (7mM) of the final composition of the...
Embodiment 3
[1208] Example 3: Image-guided high-flow CED in recurrent glioblastoma (RGBM)
[1209] Initial experience with the Phase 2 study of the targeted immunotherapy PRX 321 (CPIL-4PE)
[1210] Introduction: PRX 321 is a targeted immunotherapeutic comprising a circular arrangement of interleukin-4 fused to a truncated version of Pseudomonas exotoxin A (PE). PRX 321 binds to the interleukin-4 receptor overexpressed by glioblastoma cells and immunosuppressive cells of the tumor microenvironment, and is endocytosed by the cleaved PE domain, thereby inducing tumor cell induction via ADP-ribosylation of elongation factor 2 die.
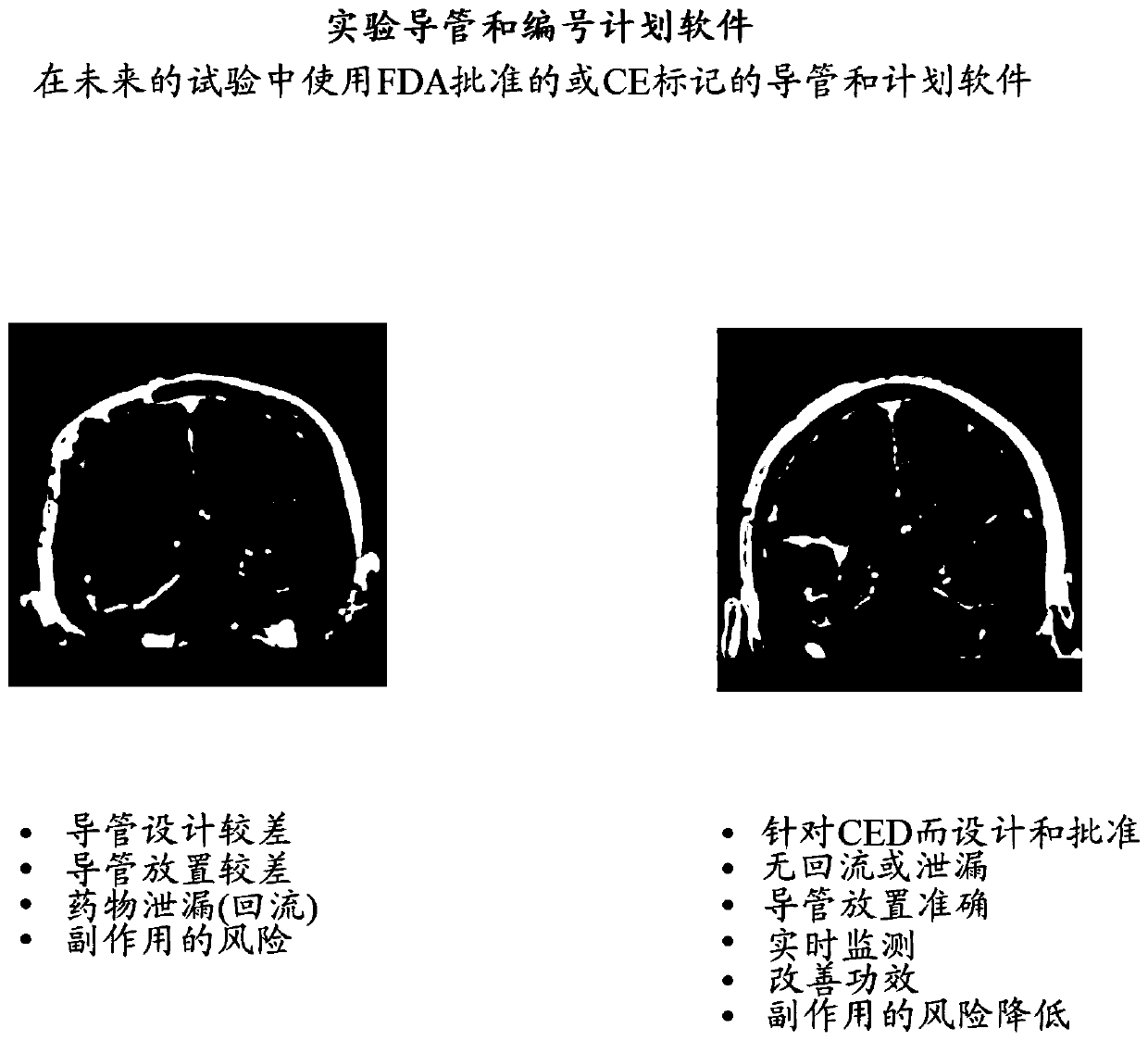
[1211] Methods: The current study is a multicentre, single-arm, 2b intratumoral infusion of PRX 321 in rGBM using staged catheters, infusion modeling (for catheter placement), and intraoperative real-time imaging of drug distribution. period study. Infusions were started at 3 μL / min / catheter and then gradually increased under real-time MRI imaging based on obs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com