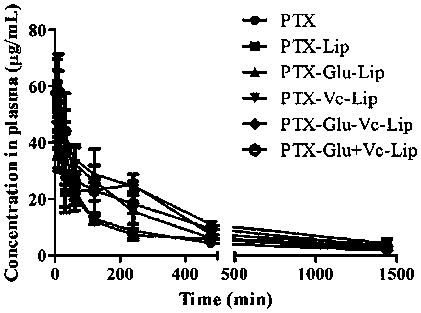
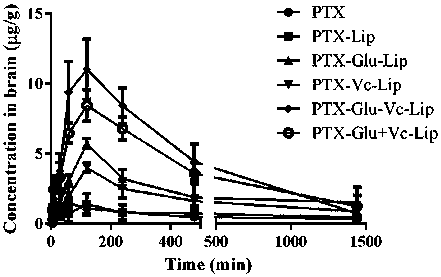
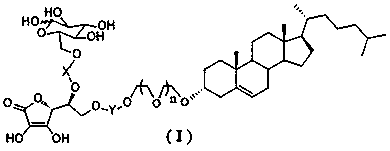
Novel dual brain-targeting lipid material and application thereof in drug delivery system
A lipid material and brain-targeting technology, applied in the field of medicine, can solve the problems of limited targeting ability and limited improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of Compound 2
[0042]
[0043] Add succinic anhydride 1 (5.00 g, 49.96 mmol), benzyl alcohol (5.94 g, 54.96 mmol) and 4-dimethylaminopyridine (DMAP, 61 mg, 0.50 mmol) into 50 mL of tetrahydrofuran, heat up to 50 °C The reaction was stirred for 5 hours. Remove the solvent under reduced pressure, add 100 mL of ethyl acetate to the residue, wash with saturated sodium bicarbonate (100 mL), discard the organic layer, adjust the aqueous layer to pH = 2 with dilute hydrochloric acid (1 mol / L), and filter , the filter cake was dried to obtain 6.58 g of white solid, yield 63.29%, Mp:60-62 o c.
Embodiment 2
[0045] Preparation of Compound 4
[0046]
[0047] Anhydrous glucose 3 (Glu, 18.02 g, 0.10 mol) was dissolved in 230 mL of anhydrous pyridine, and after cooling in an ice bath for 5 minutes, trimethylchlorosilane (TMSCl, 76.06 mL, 0.60 mol) and hexamethyl A mixed solution of disilazyl amine (HDMS, 62.88 mL, 0.30 mol) was slowly added dropwise to the above pyridine solution, and stirred at room temperature for 24 hours. Remove the solvent under reduced pressure, add 200 mL of water to disperse, extract the aqueous layer with diethyl ether (200 mL × 2), combine the organic layers, and successively wash with dilute hydrochloric acid (1 mol / L, 200 mL × 2), saturated aqueous sodium chloride (200 mL × 2) Wash, dry over anhydrous sodium sulfate, and remove the solvent under reduced pressure to obtain 52.87 g of yellow oil, with a yield of 97.70%. The product can be directly subjected to the next reaction without purification.
Embodiment 3
[0049] Preparation of compound 5
[0050]
[0051] Compound 4 (10.99 g, 20.31 mmol) was dissolved in a mixed solution of acetone and methanol (5:8, 65 mL), and acetic acid (2.1 mL, 36.72 mmol) in acetone and methanol (5:8) was slowly added dropwise under ice cooling. 8, 6.5 mL) mixed solution. After the dropwise addition, the reaction solution was moved to room temperature and stirred for 2 hours, and sodium carbonate powder (3.30 g, 31.14 mmol) was added to continue stirring at room temperature for 20 minutes. The white solid was removed by filtration, the filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate=50 / 1) to obtain 7.40 g of a colorless oil with a yield of 77.65%. 1 H NMR (400 MHz, CDCl 3 , ppm) δ : 0.12-0.18 (m, 36 H), 3.31-0.34 (m, 1 H), 3.37 (dd, 1 H, J = 2.8 Hz, 9.2 Hz), 3.55(t, 1 H, J = 8.8 Hz), 3.62-3.64 (m, 3H), 3.79 (t, 1 H, J = 8.8 Hz), 5.02 (d,1 H, J =...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com