Application of heat-resistant beta-glucosidase in preparation of gentiooligosaccharide
A technology of glucosidase and gentiosaccharide oligosaccharide, applied in the application, glycosylase, enzyme and other directions, can solve the problems of little research on β-glucosidase and the application prospect is yet to be developed, etc., and achieves high thermal stability, Efficient expression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
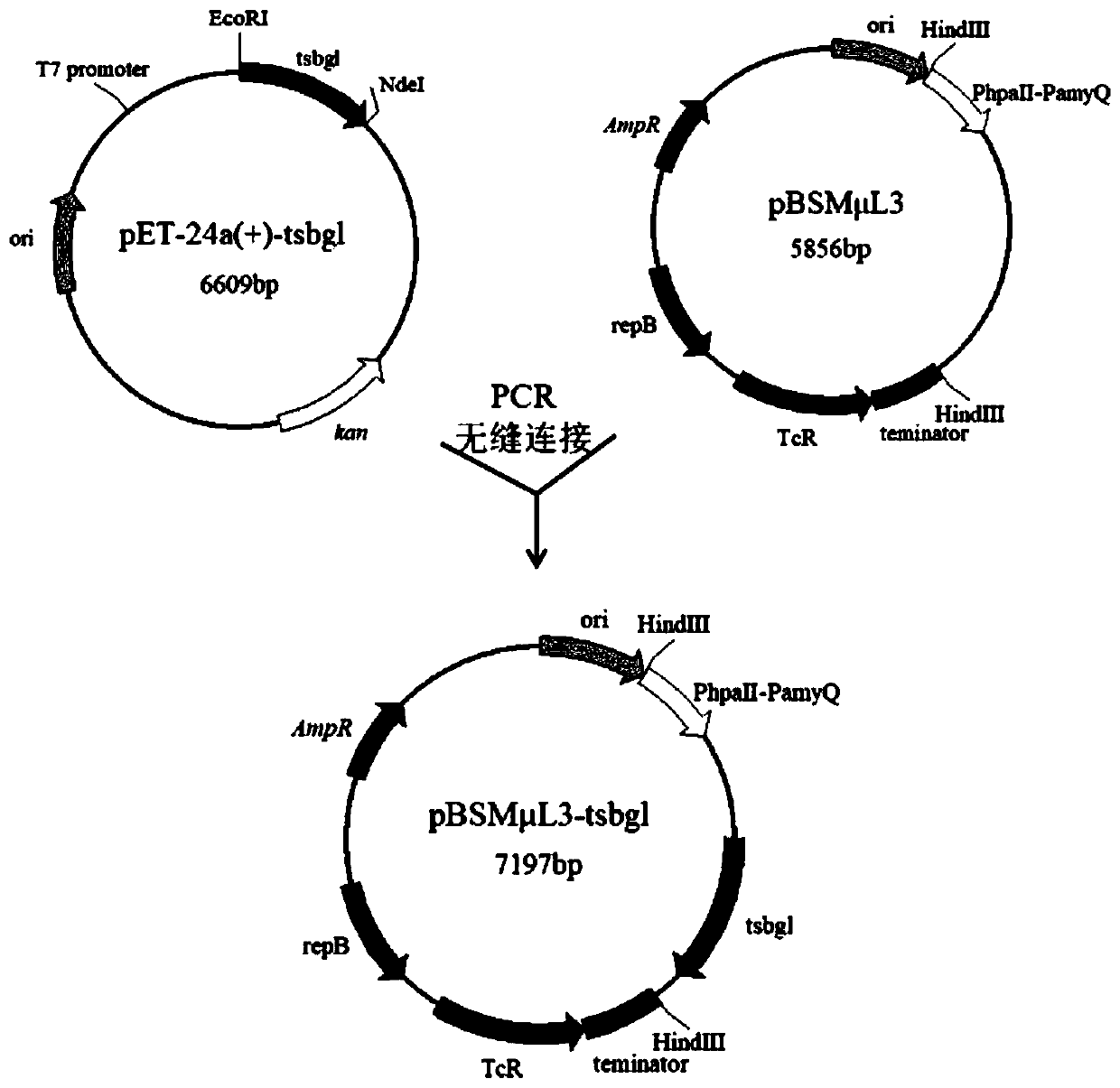
[0054] Embodiment 1: Contain the construction of tsbgl gene expression vector
[0055] According to the amino acid sequence of Thermotoga spKOL6 β-glucosidase Tsbgl (WP_101510358) in the Genbank database, the gene whose nucleotide sequence is shown in SEQ ID NO.1 was chemically synthesized.
[0056] The synthetic gene fragment was digested with pET-24a (restriction sites: Nde I and EcoR I) to obtain a ligation product; the ligation product was transformed into E. coli E. coli. JM109 by heat shock transformation method. The transformation product was obtained; the transformation product was coated on LB solid medium (containing 0.05 mg / mL kanamycin), and cultured upside down in a constant temperature incubator at 37°C for 8-12 hours to obtain a transformant.
[0057] Heat shock conversion method:
[0058] (1) Put E.coli.JM109 competent cells on ice for 5 minutes in advance. After the competent cells are completely melted, add 10 μL of complete plasmid or PCR product to them, b...
Embodiment 2
[0071] Example 2: Bacillus subtilis expression host transformation culture and extraction of crude enzyme solution
[0072] The recombinant plasmid pBSMμL3-tsbgl, which was verified by enzyme digestion and sequenced correctly, was linearized and then electrotransformed into Bacillus subtilisWSH11 (Bacillus subtilisWSH11 is described in the patent publication number CN108102997A). The recombinant plasmid pBSMμL3-tsbgl was electroporated to transform Bacillus subtilisWSH11 competent cells:
[0073] (1) Place the Bacillus subtilisWSH11 competent cells on ice for 5 minutes in advance, and after the competence is completely melted, add 10 μL of recombinant plasmid to them, blow gently and evenly, and place on ice for 15 minutes;
[0074] (2) Turn on the electroporator to preheat for 30 minutes in advance, and set the electric shock voltage to 2400V. Slowly add the competent state after the ice bath to the electric shock cup with a diameter of 2 mm for extraction and pre-cooling. Af...
Embodiment 3
[0080] Embodiment 3: the determination of β-glucosidase TSBG1 application condition
[0081] (1) Optimal temperature of β-glucosidase TSBG1
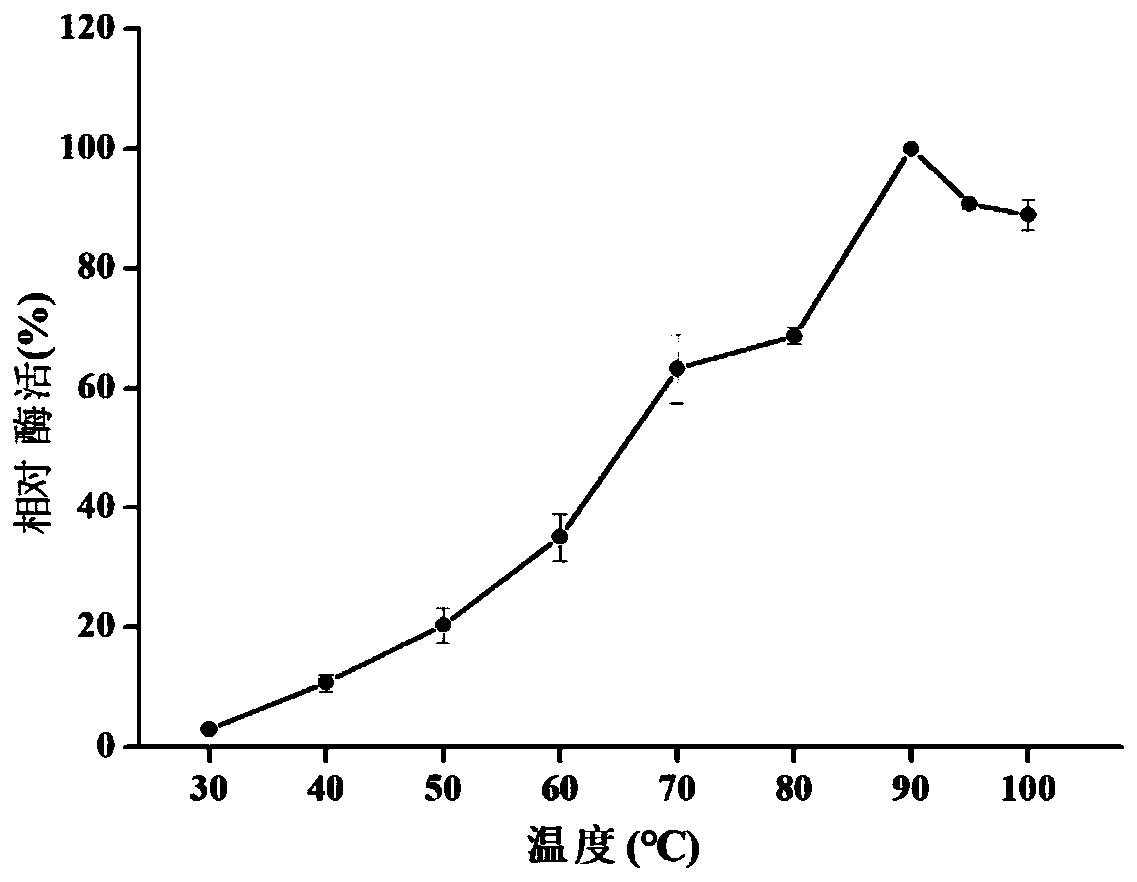
[0082] Using pNPG as a substrate, add the purified β-glucosidase obtained in Example 3 into the enzyme activity assay reaction system, the pH is 6.0, react at different temperatures, measure the enzyme activity, and calculate its relative enzyme activity , see the result image 3 , the specific data are shown in Table 1, it can be seen that the optimum temperature of β-glucosidase is 90°C, and at 90-100°C, the relative enzyme activity can reach more than 85%.
[0083] The relative enzyme activity of table 1β-glucosidase TSBG1 under different temperatures
[0084]
[0085] (2) Optimum pH of β-glucosidase TSBG1
[0086] Using pNPG as a substrate, the purified β-glucosidase obtained in Example 3 was added to the enzyme activity assay reaction system, and reacted in a constant temperature water bath at 90° C. for 10 minutes at differen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com