Application of mitochondrial transplantation in treatment of primary dilated heart disease
A mitochondrial, primary technology, applied in cardiovascular system diseases, medical preparations containing active ingredients, unknown raw materials, etc. Cardiac cavity dilation and improvement of cardiac dysfunction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
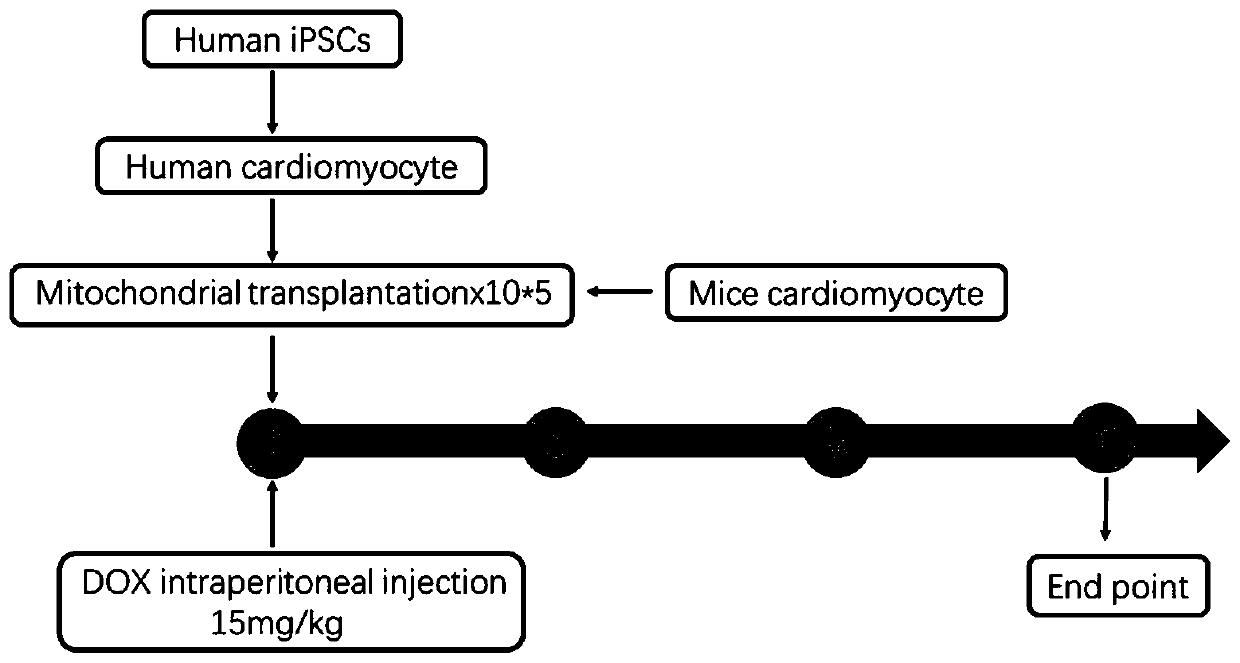
[0039] According to the experimental road map of the present invention, see figure 1 , follow the steps below:
[0040] Matrigel Coated Petri Dishes
[0041] 1.1 Matrigel (BD, bioscience) is aliquoted and stored at -20°C / -80°C, and the aliquoted Matrigel is thawed at 4°C overnight before use.
[0042] 1.2 Pre-cool Matrigel and DMEM+F12 medium at 4°C, dilute according to the ratio of 800μl / 50ml and mix immediately. This process should be performed quickly to prevent the gel from solidifying. Add 1ml of medium to each well of the 6-well plate.
[0043] 1.3 Seal the culture dish with a parafilm to prevent evaporation, and put it in a 37°C cell incubator for at least 2 hours before using it for subsequent experiments. If it is not used temporarily, store it at 4°C.
[0044] 1.4 Before using the Matrigel-coated 6-well plate, remove the medium in the well plate and add the medium to be used.
[0045] 2. Culture and passage of IPS cells
[0046] 2.1 When the cell density reaches ...
Embodiment 2
[0061] The animal tissue and cell mitochondria preparation methods were operated on ice according to the requirements of the Beyontian animal tissue and cell mitochondria separation kit used, the lysis conditions of the lysate were kept the same, and the number of grinding rods was 40-50 times.
[0062] After the mitochondria were prepared, they were dissolved in PBS and stored on ice. A part of the mitochondrial solution was drawn, diluted and counted to adjust the concentration of the mitochondrial working suspension. Mitochondria were used immediately after preparation and transplanted into animal myocardium within 1 hour. The longer the mitochondria are kept in vitro, the poorer their viability.
Embodiment 3
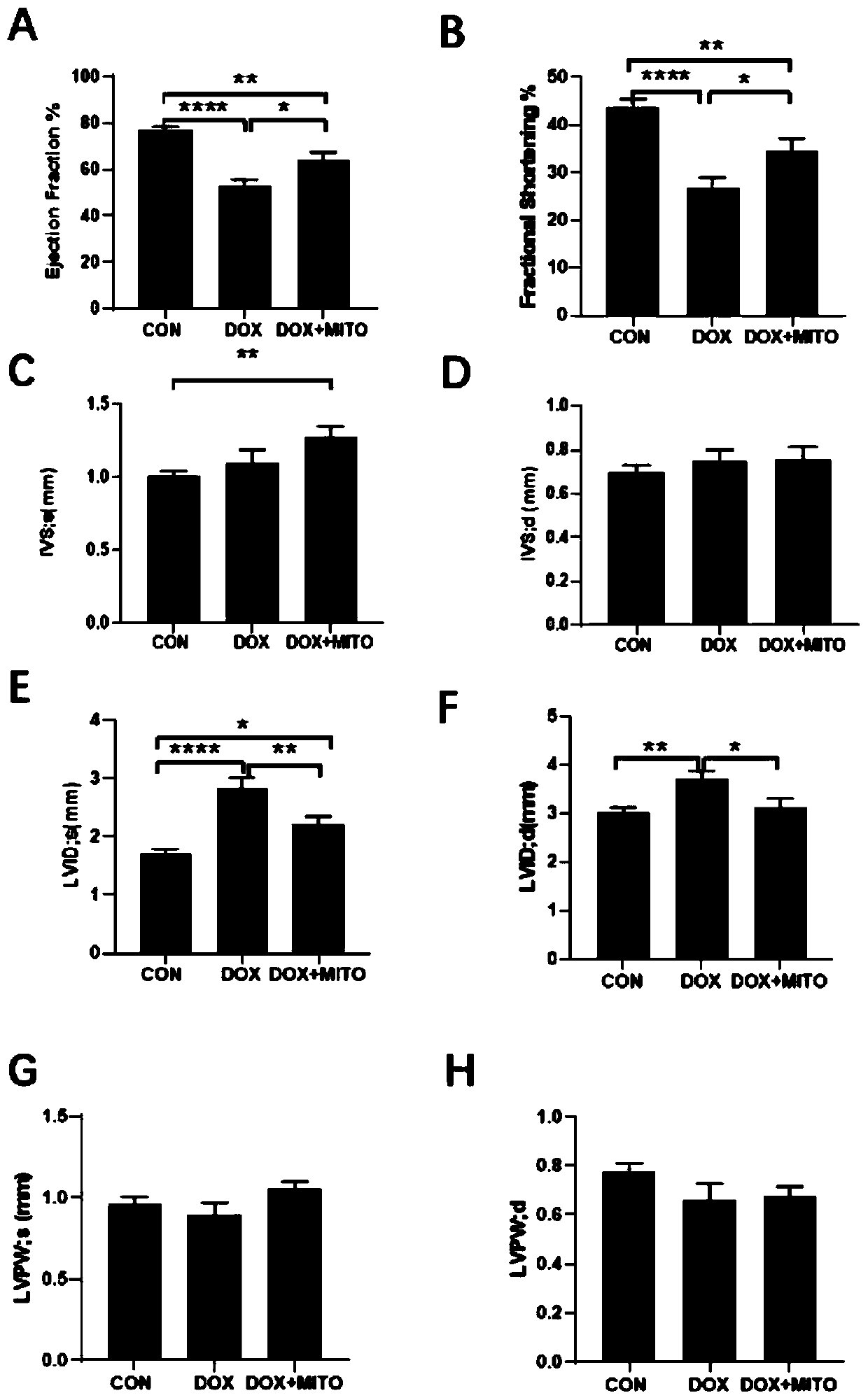
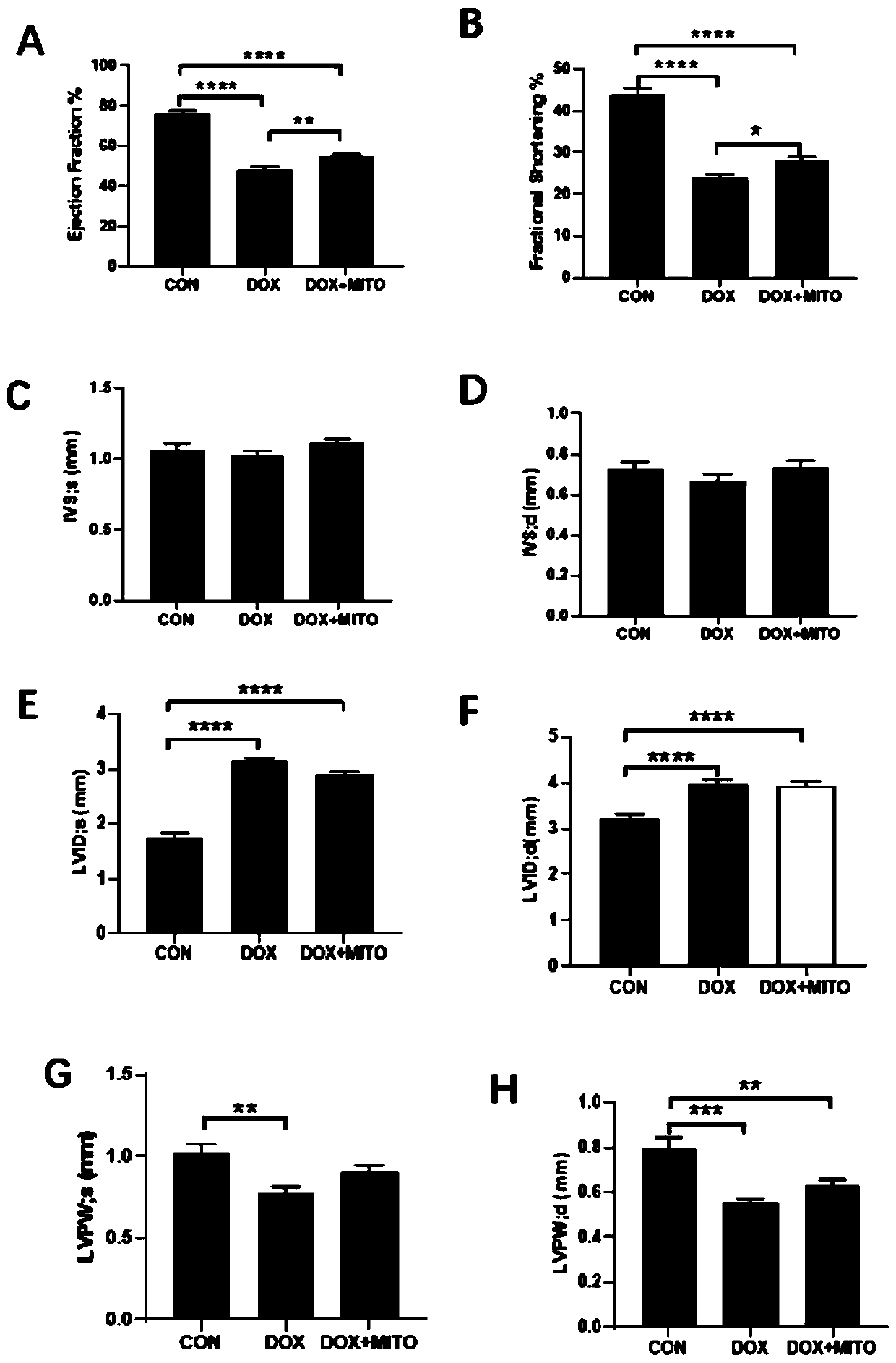
[0064] Three groups were set up in the experiment: Sham group, DCM group, DCM+mitochondria transplantation group. Adult C57BL / 6 mice were depilated in the chest in advance, anesthetized with 2% isoflurane gas inhalation, and left thoracotomy was performed to separate the pectoralis major from the pectoralis minor, and the pleura and pericardium were opened with hemostats. Extrude the heart and transplant 100 μl at multiple points on the surface of the left ventricle of the myocardium (each 100 μl contains 10 5 mitochondria) normal saline suspension, and intraperitoneal injection of 15 mg / kg doxorubicin at the same time; the same volume of PBS was injected intraperitoneally in the DCM group, and 15 mg / kg doxorubicin was injected intraperitoneally; in the Sham group, only the heart was extruded by thoracotomy, and the same volume of doxorubicin was injected intramuscularly. PBS, no intraperitoneal injection of doxorubicin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com