Frovatriptan tablet and preparation method thereof
A technology for furotriptan tablets and furotriptan, which can be applied to pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve the problems of affecting the rest of patients and unsatisfactory treatment effects. , to improve the quality of rest, relieve vomiting and nausea, and reduce side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
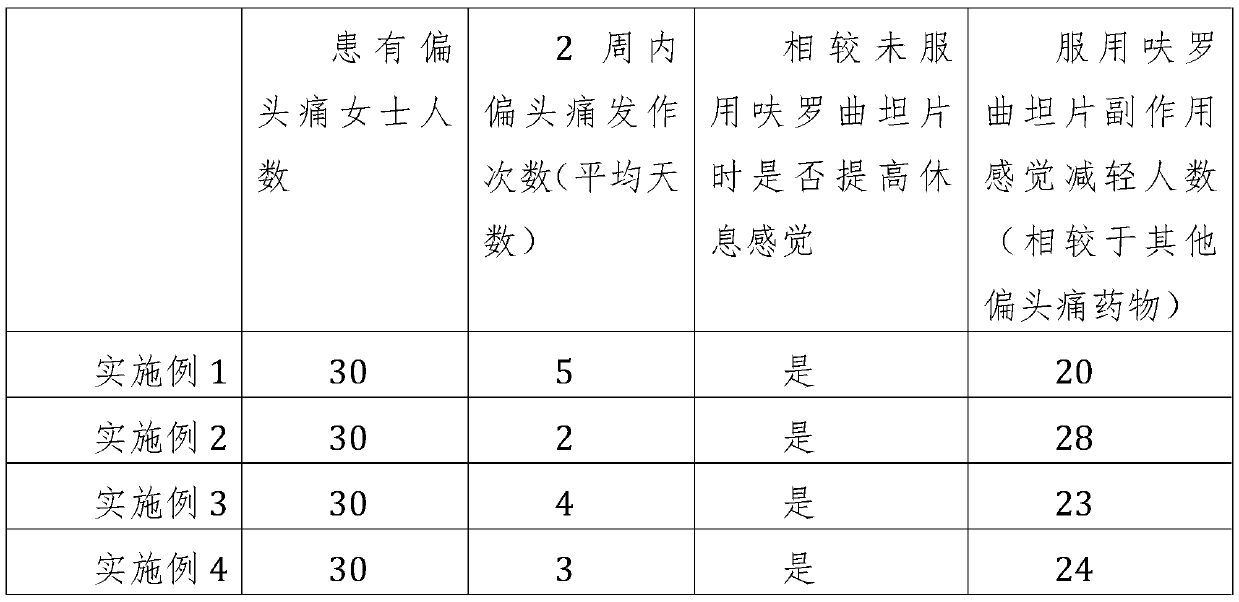
Examples
Embodiment 1
[0024] A kind of furotriptan tablet, wherein used major ingredient comprises the raw material of following parts by weight: 150 parts of furotriptan succinate, 50 parts of propranolol, 50 parts of amitriptyline, hypromellose 1000 parts, 800 parts of pregelatinized starch, 100 parts of wetting agent, 40 parts of wild chrysanthemum, 40 parts of night vine, 40 parts of skullcap, 40 parts of lotus seeds, 500 parts of edible gelatin.
[0025] Further, the wetting agent is selected as 95% ethanol solution.
[0026] Further, the said wild chrysanthemum, night vine, scutellaria baicalensis and lotus seeds are all ground into powder.
[0027] A furovatriptan tablet also includes a preparation method of the furovatriptan tablet:
[0028] Step 1: Take furovatriptan succinate, propranolol, amitriptyline, hypromellose, and pregelatinized starch according to the corresponding number of prescriptions. After screening, mix evenly, and then add a wetting agent for Stir evenly to make soft me...
Embodiment 2
[0036] A kind of furotriptan tablet, wherein used major ingredient comprises the raw material of following parts by weight: 200 parts of furotriptan succinate, 65 parts of propranolol, 65 parts of amitriptyline, hypromellose 1500 parts, 1000 parts of pregelatinized starch, 125 parts of wetting agent, 55 parts of wild chrysanthemum, 55 parts of night vine, 55 parts of skullcap, 55 parts of lotus seeds, 650 parts of edible gelatin.
[0037] Further, the wetting agent is selected as 95% ethanol solution.
[0038] Further, the said wild chrysanthemum, night vine, scutellaria baicalensis and lotus seeds are all ground into powder.
[0039] A furovatriptan tablet also includes a preparation method of the furovatriptan tablet:
[0040] Step 1: Take furovatriptan succinate, propranolol, amitriptyline, hypromellose, and pregelatinized starch according to the corresponding number of prescriptions. After screening, mix evenly, and then add a wetting agent for Stir evenly to make soft m...
Embodiment 3
[0048] A kind of furotriptan tablet, wherein used major ingredient comprises the raw material of following parts by weight: 250 parts of furovatriptan succinate, 80 parts of propranolol, 80 parts of amitriptyline, hypromellose 2000 parts, 1200 parts of pregelatinized starch, 150 parts of wetting agent, 70 parts of wild chrysanthemum, 70 parts of night vine, 70 parts of skullcap, 70 parts of lotus seeds, 800 parts of edible gelatin.
[0049] Further, the wetting agent is selected as 95% ethanol solution.
[0050] Further, the said wild chrysanthemum, night vine, scutellaria baicalensis and lotus seeds are all ground into powder.
[0051] A furovatriptan tablet also includes a preparation method of the furovatriptan tablet:
[0052] Step 1: Take furovatriptan succinate, propranolol, amitriptyline, hypromellose, and pregelatinized starch according to the corresponding number of prescriptions. After screening, mix evenly, and then add a wetting agent for Stir evenly to make soft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com