Preparation method of HBx protein derived from Hepatitis B Virus HBV
A protein and mercaptoethanol technology, applied in the biological field, can solve the problems of inability to obtain high-purity, stable and uniform protein samples, low solubility, and failure to remove nucleic acids.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
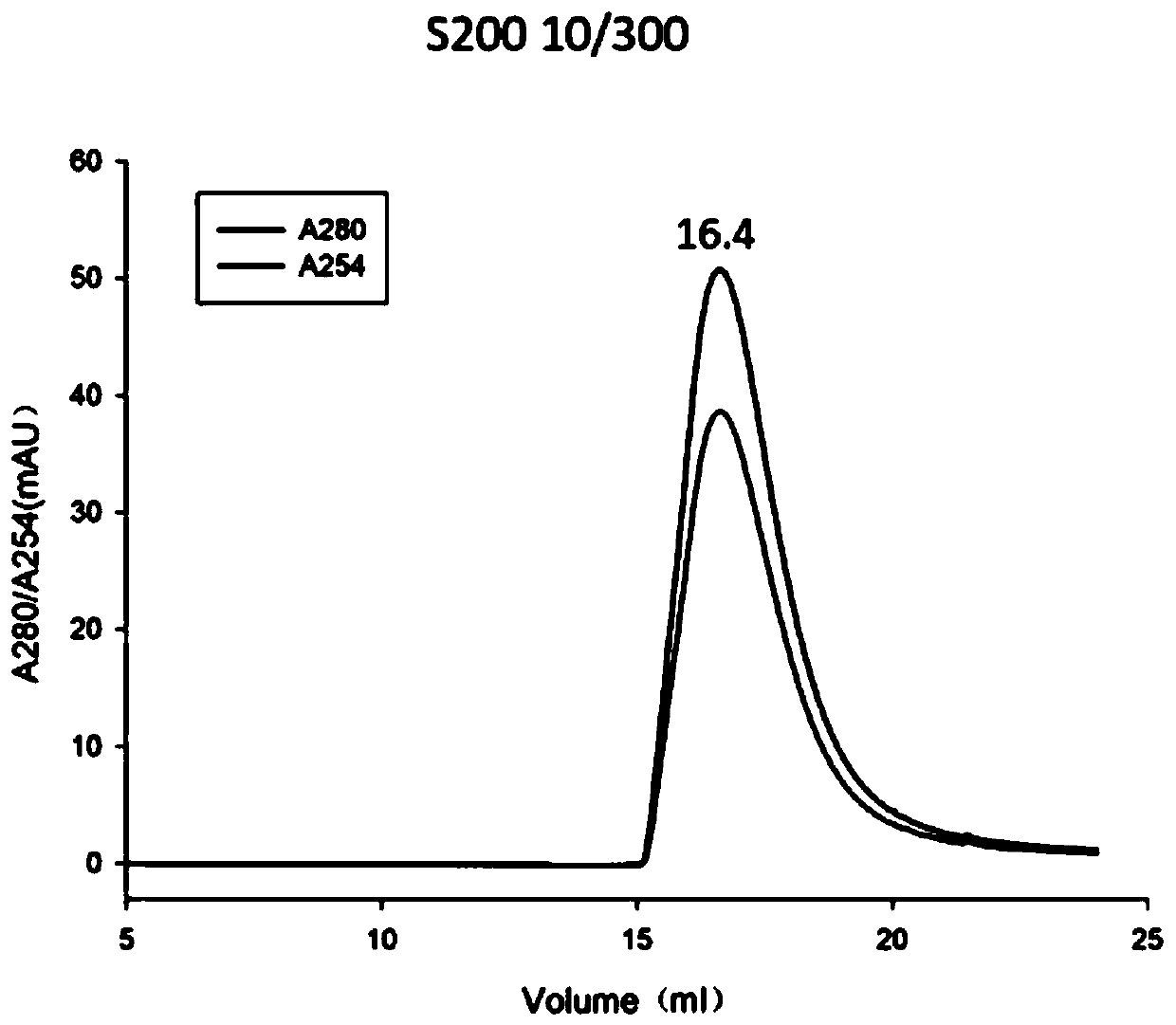
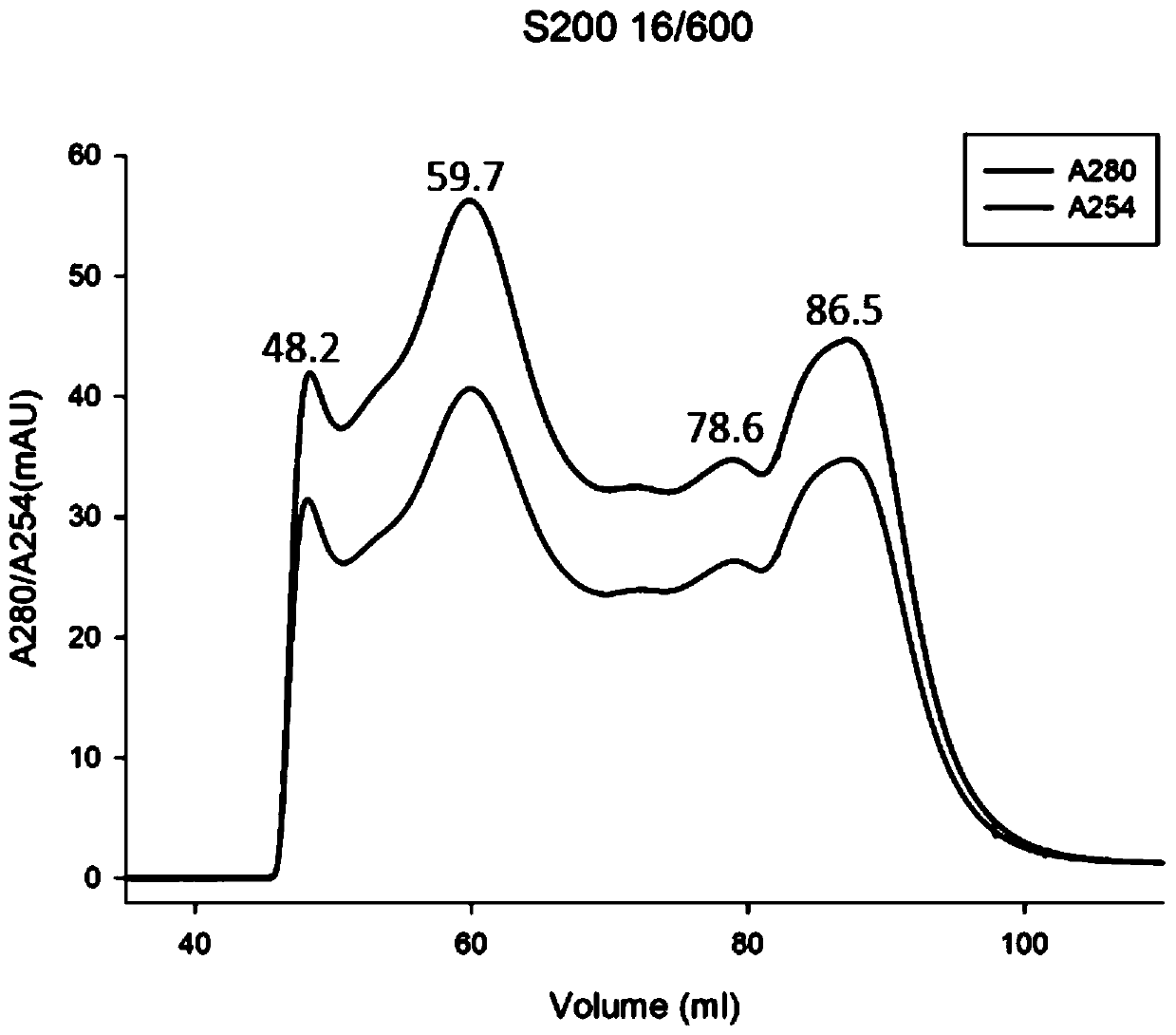
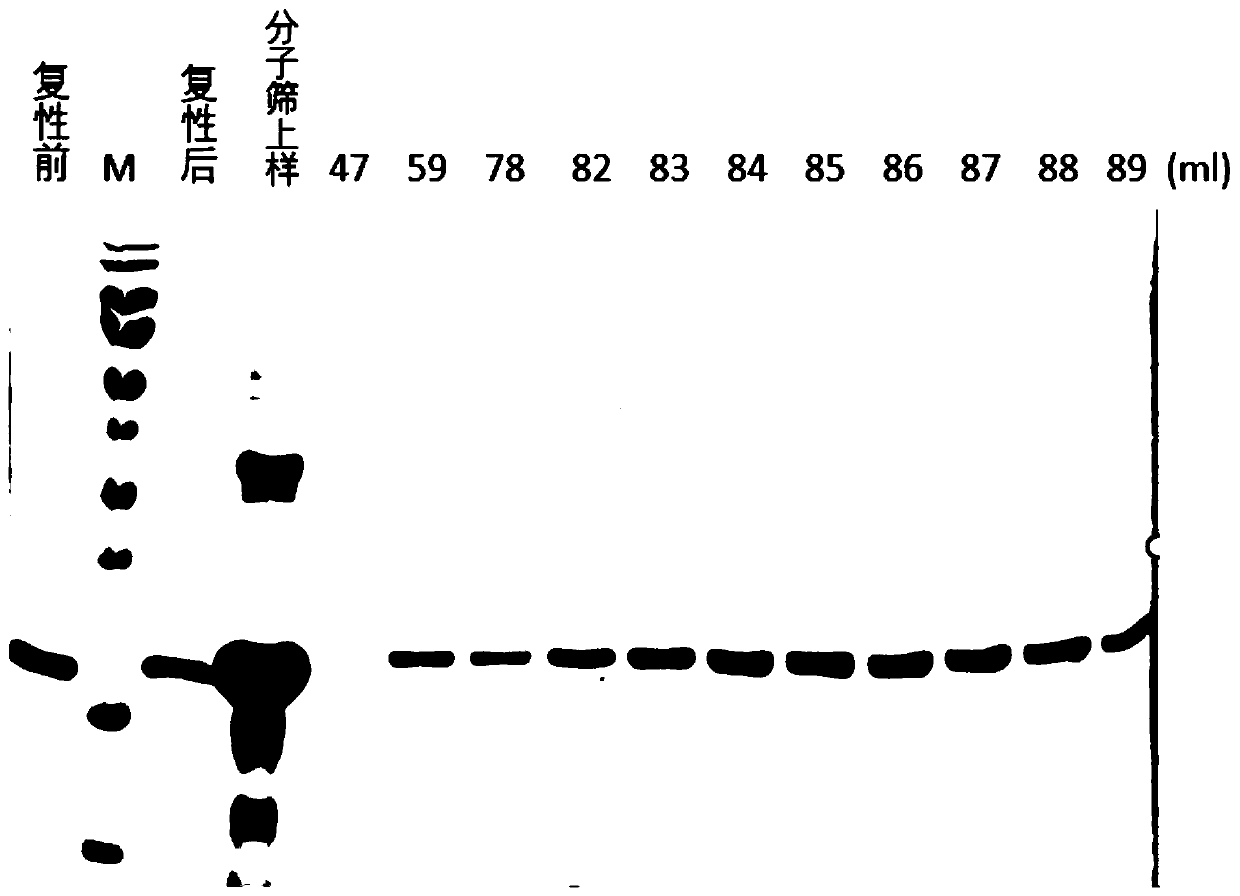
[0044] Example 1 Solubilization and purification of HBx inclusion bodies
[0045] In the present invention, the full-length sequence of HBx is cloned on the pet15b expression vector, the endonuclease NcoI and XhoI restriction sites are selected, 6×His at the N-terminal of the vector is retained, and 6 His can also be added at the C-terminal of the vector. Recombinant proteins with only N-terminal 6×His and both N and C-terminal 6×His can be used for subsequent solubilization and purification.
[0046] In the present invention, the prokaryotic cell expression system of Escherichia coli is preferably used (but other expression systems are not excluded, such as expression in other bacteria or other eukaryotic cells); the above-mentioned proteins are expressed in the form of inclusion body proteins , the His tag is preferred (but other tags, such as MBP, GST or SUMO tags are not excluded).
[0047] Specifically, the present invention transforms the plasmid containing the HBx sequ...
Embodiment 2
[0056] Example 2 Preparation of HBx monoclonal antibody
[0057] Using the HBx sample obtained by the above purification method as an antigen, the HBx monoclonal antibody can be obtained by using the usual monoclonal antibody preparation method. E.g:
[0058] The HBx sample obtained by the above purification method is used as an antigen and injected into mice to prepare mouse-derived HBx monoclonal antibody, and obtain a hybridoma cell line capable of stably producing mouse-derived HBx monoclonal antibody.
[0059] Using the HBx obtained by the above purification method as an antigen, injecting other animals than mice can prepare monoclonal antibodies from other animals.
[0060] Humanized HBx monoclonal antibodies can also be obtained by using the above-mentioned animal-derived (such as mouse-derived) monoclonal antibodies using common antibody humanization methods.
[0061] The sequence used in the present invention is as follows:
[0062] SEQ.ID.NO1:
[0063] MAARVCCQLD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com