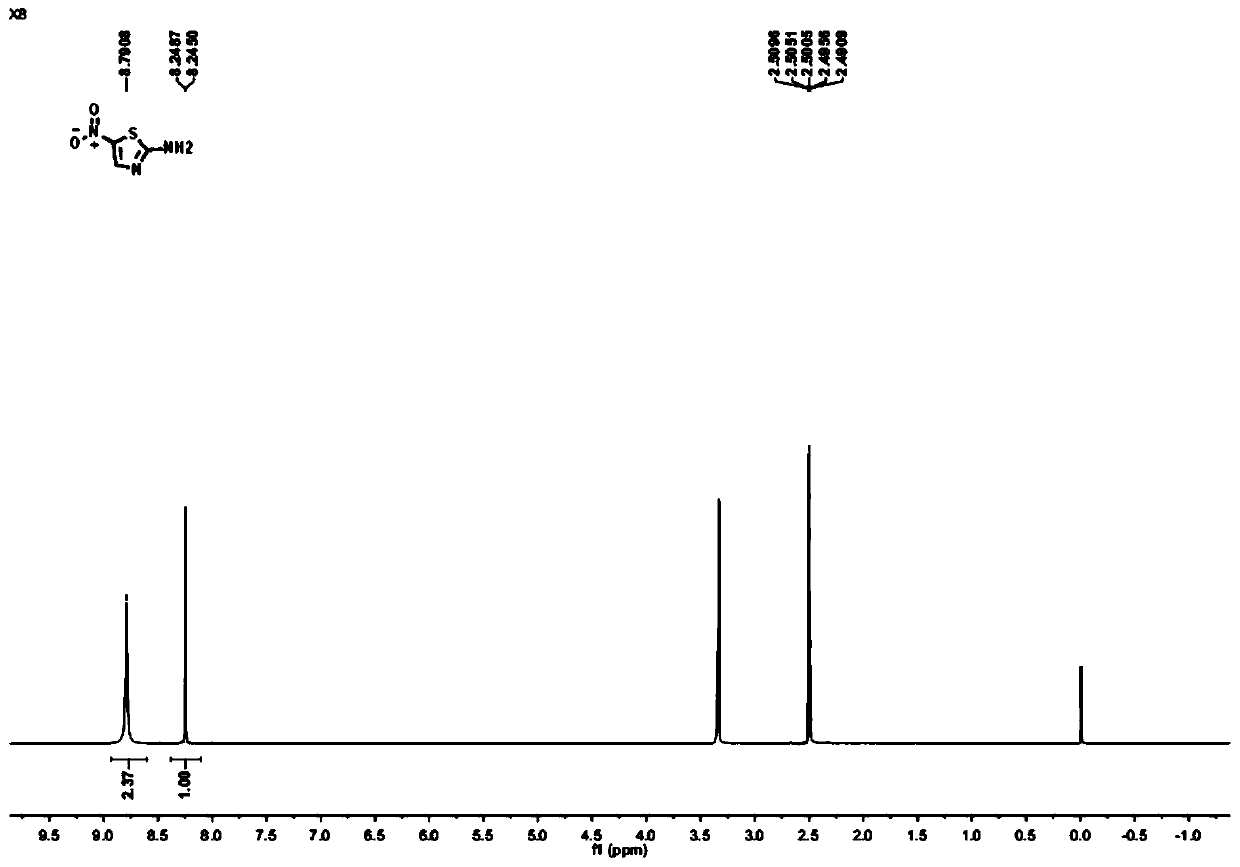
Method for synthesizing 2-amino-5-nitrothiazole
A technology of nitrothiazole and dimethylnitroethylene, which is applied in the field of synthesizing 2-amino-5-nitrothiazole, can solve the problems of high cost, difficult to obtain raw materials, low yield, etc., achieve mild reaction conditions, solve Raw materials are expensive and the effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
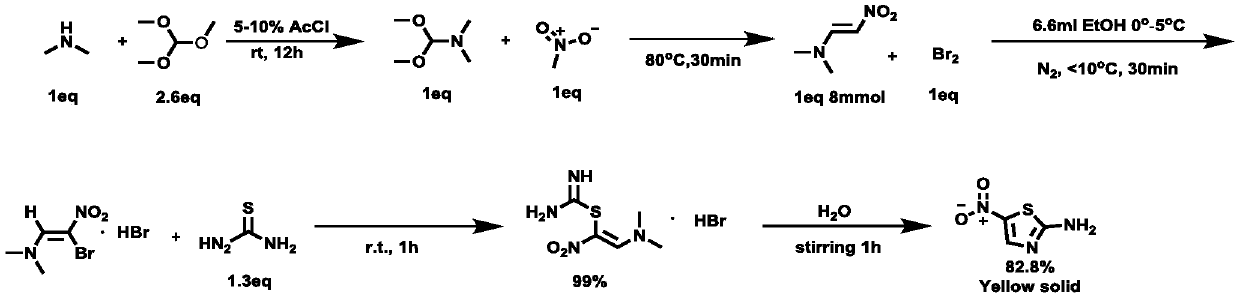
[0038] (1) Under an argon atmosphere, according to the molar ratio of 1:0.2:2.6, add diethylamine, acetyl chloride, and triethyl orthoacetate into the reaction vessel and mix well;
[0039] (2) The reaction vessel was placed on a room temperature stirrer and stirred vigorously for 12 hours, and the reaction product was purified by distillation to obtain N,N-dimethylformamide dimethyl acetal with a yield of 95%;
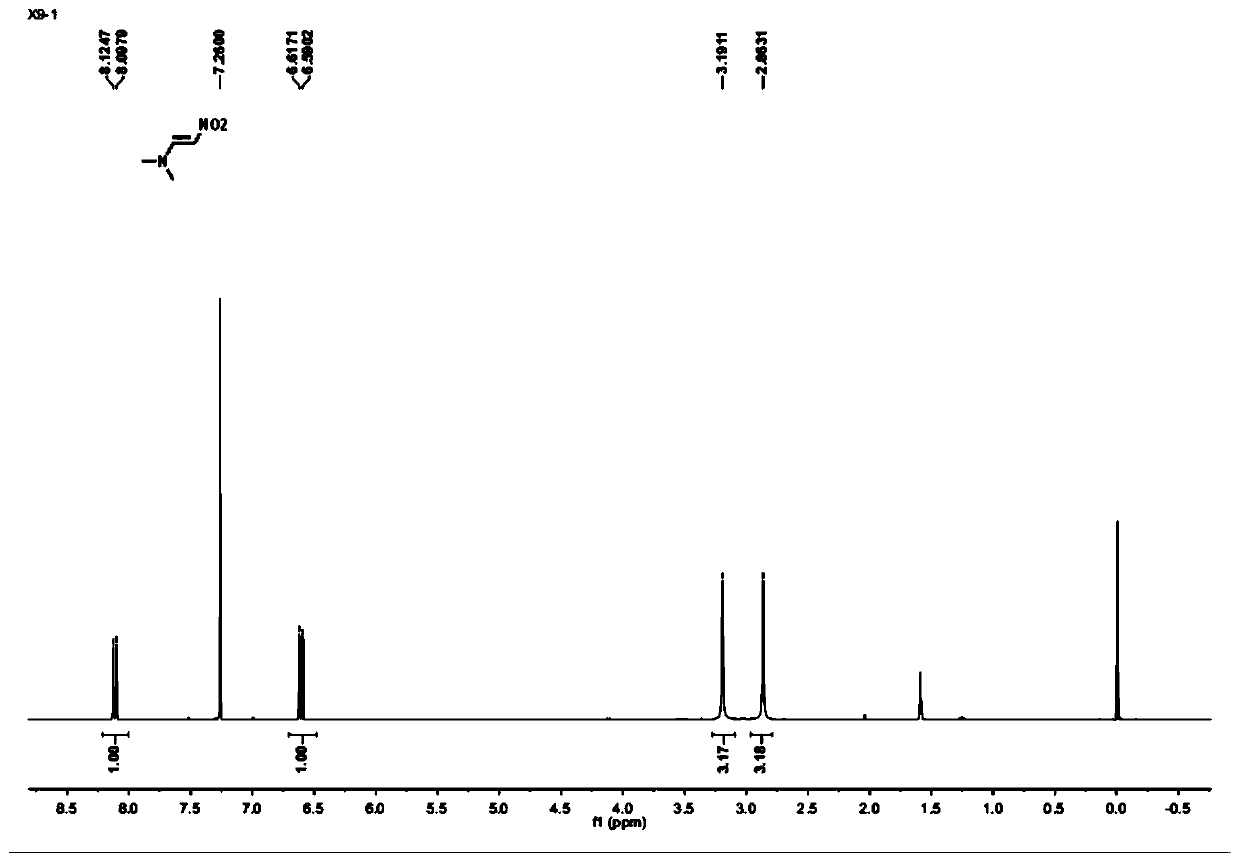
[0040] (3) Under an argon atmosphere, add the N,N-dimethylformamide dimethyl acetal obtained in step (2) into nitromethane, and heat the mixture to 80° C. for reflux reaction for 30 minutes; wherein, the The molar ratio of N,N-dimethylformamide dimethyl acetal to nitromethane is 1:1; the reaction product is distilled off under reduced pressure to remove the solvent, and the reaction product is purified by washing the chromatographic silica gel column with ethyl acetate to obtain N , N-dimethylnitroethylene;
[0041] (4) Under an argon atmosphere, put the N,N-dimethyl...
Embodiment 2
[0046] This embodiment is basically the same as Embodiment 1, the difference is:
[0047] In step (4), the molar ratio of N,N-dimethylnitroethylene, liquid bromine, ethanol, thiourea and water is 1:1:10:1.3:23.
Embodiment 3
[0049] This embodiment is basically the same as Embodiment 1, the difference is:
[0050] In step (4), the molar ratio of N,N-dimethylnitroethylene, liquid bromine, ethanol, thiourea and water is 1:1:7:1.3:40.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com