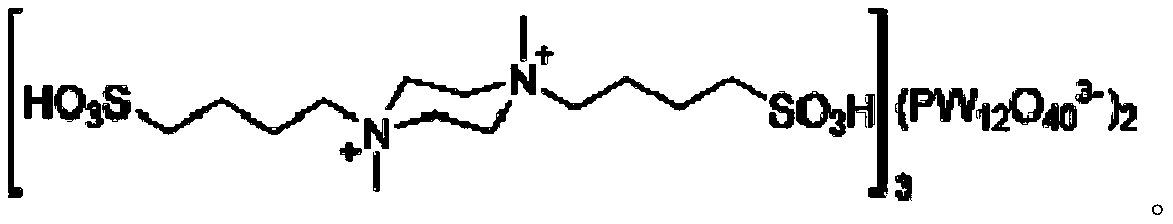
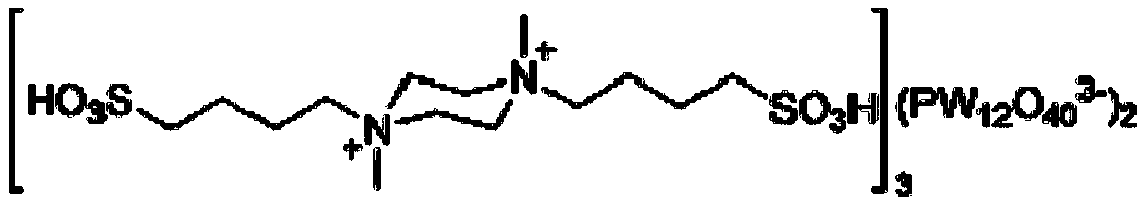
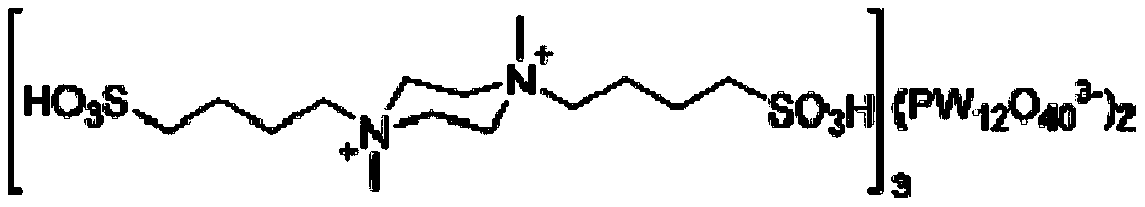
Method for catalytically preparing chromene pyrimido indazolone derivative serving as drug intermediate
A technology for catalytic preparation and intermediates, which is applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve the problems of large catalyst usage and complicated purification process, and achieve the reduction of waste liquid discharge, Overcome the effect of complex purification process and simple purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Add 1.0mmol benzaldehyde, 1.0mmol 1H-indazole to a 50ml three-necked flask with a spherical condenser, a thermometer and a magnetic stirrer that contains 6ml of ethanol aqueous solution (the percentage of ethanol volume accounting for the total volume of ethanol-distilled water is 78%) -3-amine and 1.0mmol 4-hydroxycoumarin, stirred at room temperature, mixed evenly, and then added 0.03mmol acidic ionic liquid catalyst. Heat in an oil bath, evenly heat up to solvent reflux (solvent vapor does not exceed the second ball of the spherical condenser), keep reflux for 6 minutes, TLC (thin plate chromatography) detection, the raw material point disappears, and the reaction is over. Turn off the heating and stirring, the reaction solution is naturally cooled to room temperature, and a large amount of solids are precipitated, crushed and left to stand for 12 hours, filtered under reduced pressure, the filter residue is washed with absolute ethanol (5ml×3), and vacuum-dried at 80...
Embodiment 2
[0051] Add 1.0mmol p-chlorobenzaldehyde, 1.0mmol1H-indole to a 50ml three-necked flask with a spherical condenser, a thermometer and a magnetic stirrer that contains 7ml of ethanol aqueous solution (the percentage of ethanol volume accounting for the total volume of ethanol-distilled water is 81%) Azol-3-amine and 1.0 mmol of 4-hydroxycoumarin were stirred at room temperature and mixed uniformly, and then 0.04 mmol of acidic ionic liquid catalyst was added. Heat in an oil bath, evenly heat up to solvent reflux (solvent vapor does not exceed the second ball of the spherical condenser), keep reflux for 7 minutes, TLC (thin plate chromatography) detection, the raw material point disappears, and the reaction is over. Turn off the heating and stirring, the reaction solution is naturally cooled to room temperature, and a large amount of solids are precipitated, crushed and left to stand for 12 hours, filtered under reduced pressure, the filter residue is washed with absolute ethanol ...
Embodiment 3
[0056] Add 1.0mmol p-bromobenzaldehyde, 1.0mmol1H-indole to a 50ml three-necked flask with a spherical condenser, a thermometer and a magnetic stirrer that contains 7ml of ethanol aqueous solution (the percentage of ethanol volume accounting for the total volume of ethanol-distilled water is 81%) Azol-3-amine and 1.0 mmol 4-hydroxycoumarin were stirred at room temperature and mixed uniformly, and then 0.05 mmol of acidic ionic liquid catalyst was added. Heat in an oil bath, evenly heat up to solvent reflux (solvent vapor does not exceed the second ball of the spherical condenser), keep reflux for 8 minutes, TLC (thin plate chromatography) detection, the raw material point disappears, and the reaction is over. Turn off the heating and stirring, the reaction solution is naturally cooled to room temperature, and a large amount of solids are precipitated, crushed and left to stand for 12 hours, filtered under reduced pressure, the filter residue is washed with absolute ethanol (5ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com