Preparation method of m-nitrobenzotrifluoride
A technology of nitrobenzotrifluoride and trifluorotoluene, which is applied in the field of preparation of m-nitrobenzotrifluoride, can solve the problems of tediousness and post-explosion treatment, achieve low risk, reduce violent heat release, and solve cumbersome post-treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
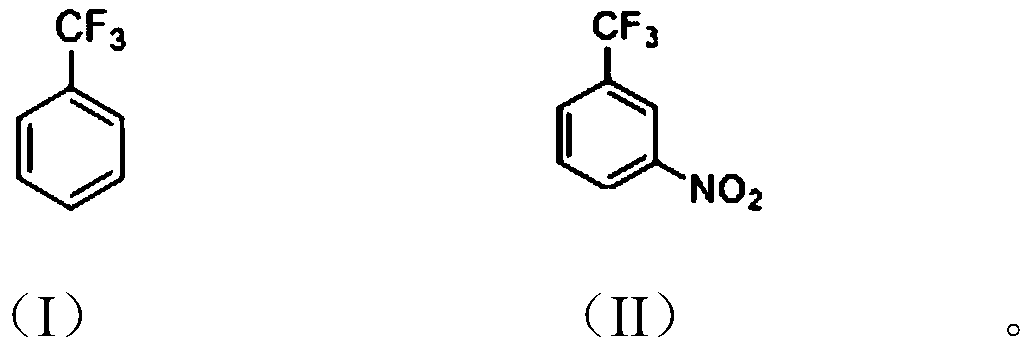
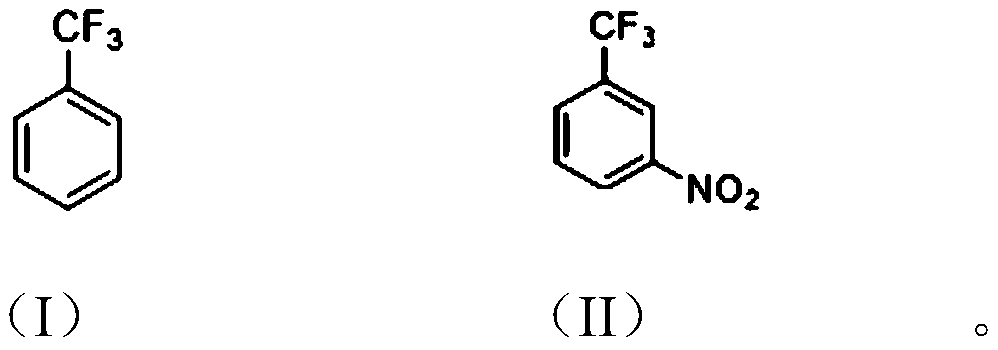
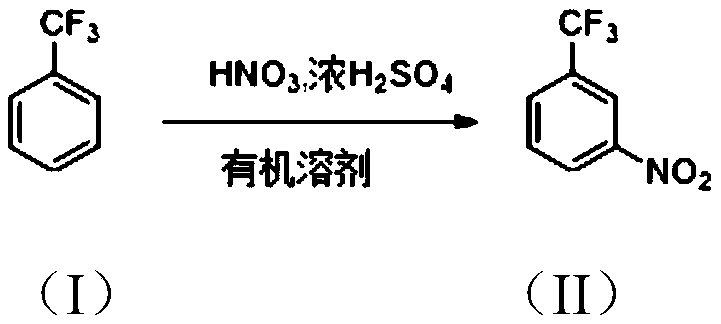
[0024] The invention provides a method for preparing m-nitrobenzotrifluoride, which uses an organic solvent to participate in the preparation of m-nitrobenzotrifluoride.
[0025]
[0026] The preparation method of m-nitrobenzotrifluoride of the present invention comprises: adding trifluorotoluene shown in formula (I), organic solvent and concentrated sulfuric acid into the reaction vessel, under stirring, temperature control 30~40 ℃, dripping Smoked nitric acid, insulation reaction for 6-10 hours, after the reaction finishes, let stand to separate the liquids, the organic phase is adjusted to be alkaline with alkaline aqueous solution, the organic phase is desolvated, and finally carry out decompression distillation to obtain the formula (II) of m-nitrobenzotrifluoride.
[0027] Specifically, the organic solvent is usually a chlorinated non-polar solvent, such as dichloroethane, carbon tetrachloride, and the like. The molar ratio of trifluorotoluene to the organic solvent ...
Embodiment 1
[0039] Add 1kg of industrially pure trifluorotoluene (commercially available), 1L of dichloroethane and 336g of 98% concentrated sulfuric acid into the reaction flask, and under stirring, add 475g of fuming nitric acid dropwise at 30-40°C, dropwise After the addition, stir for 8 hours at 30-40°C, let stand, separate the liquid, remove the acid phase, wash the organic phase with aqueous sodium hydroxide solution to alkalinity, then carry out precipitation of the organic phase, and finally carry out subtractive evaporation to obtain Light yellow oily liquid m-nitrobenzotrifluoride 915g, the calculated yield is 69.8%, the purity of m-nitrobenzotrifluoride obtained by gas chromatography is 99.0%.
Embodiment 2
[0041] Add 1kg of industrially pure trifluorotoluene (commercially available), 1L of dichloroethane and 470g of 98% concentrated sulfuric acid into the reaction flask, and under stirring, add 475g of fuming nitric acid dropwise at 30-40°C. After the addition, stir for 8 hours at 30-40°C, let stand, separate the liquid, remove the acid phase, wash the organic phase with aqueous sodium hydroxide solution to alkalinity, then carry out precipitation of the organic phase, and finally carry out subtractive evaporation to obtain Light yellow oily liquid m-nitrobenzotrifluoride 1205g, the calculated yield is 92.0%, the purity of m-nitrobenzotrifluoride obtained by gas chromatography is 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com