Antifungal compound as well as synthesis method and application thereof
An antifungal and compound technology, applied in the field of drug synthesis, can solve the problems of threatening the life, health and safety of patients, interfering with fungal cell nucleic acid, protein synthesis, adverse reactions, etc., to ensure high reaction efficiency, good antifungal effect, and good reaction stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
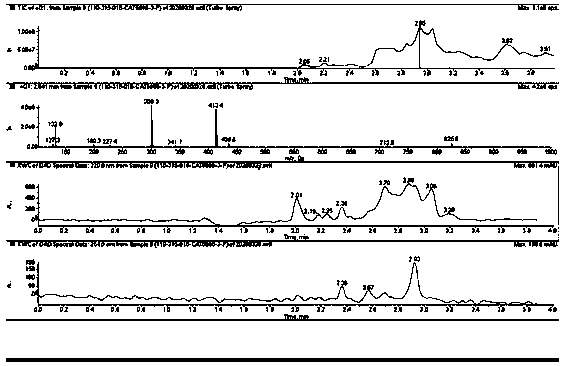
Image
Examples
Embodiment 1
[0036] A kind of antifungal compound, described antifungal compound is leucine lauryl ester, and the structural formula of described leucine lauryl ester is as follows:
[0037]
[0038] The preparation method of described leucine lauryl ester is as follows:
[0039] Dodecanol (16.8g, 0.1mol) and p-toluenesulfonic acid (20.9g, 0.1mol) were added to a solution of DL-leucine (13.1g, 0.1mol) in toluene (200mL), and the temperature was slowly raised to reflux temperature , water azeotropic separation, and the reactants were monitored by thin-layer chromatography. The reaction mixture was concentrated under vacuum and the resulting residue was extracted with ethyl acetate (200ml) and washed with 5% Na 2 CO 3 Washed with aqueous solution (3 x 50ml) and then with brine solution. in Na 2 SO 4 The organic layer was dried on top, concentrated under vacuum to get the crude product, and silica gel chromatography (MeOH:DCM=1:50) gave leucine lauryl ester (10 g, 33%) as yellow oil. ...
Embodiment 2
[0054] An antifungal compound, the antifungal compound is stearyl leucine, and the preparation method of stearyl leucine is as follows:
[0055] Add octadecyl alcohol (0.1mol) and p-toluenesulfonic acid (0.1mol) to a solution of leucine (0.1mol) in toluene (200mL), slowly heat up to reflux temperature, azeotropically separate water, and use thin layer chromatography Monitor the reactants. The reaction mixture was concentrated under vacuum and the resulting residue was extracted with ethyl acetate (200ml) and washed with 5% Na 2 CO 3 Washed with aqueous solution (3 x 50ml) and then with brine solution. in Na 2 SO 4 The organic layer was dried and concentrated in vacuo to give the crude product, which was chromatographed on silica gel (MeOH:DCM=1:5) to give leucine octadecyl ester (39%).
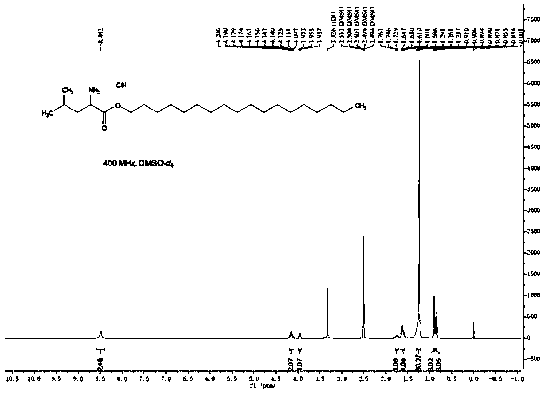
[0056] Figure 4 It is the NMR spectrum of leucine stearyl ester, through Figure 4 It can be analyzed simply, and the product is stearyl leucine.
[0057] The same experiment as the a...
Embodiment 3
[0060] A kind of antifungal compound, described antifungal compound is leucine hexaalkyl ester, the preparation method of described leucine hexaalkyl ester is as follows:
[0061] Add hexanol (0.1mol) and p-toluenesulfonic acid (0.1mol) to a solution of leucine (0.1mol) in toluene (200mL), slowly heat up to reflux temperature, azeotropically separate water, and use thin layer chromatography Reactants are monitored. The reaction mixture was concentrated under vacuum and the resulting residue was extracted with ethyl acetate (200ml) and washed with 5% Na 2 CO 3 Washed with aqueous solution (3 x 50ml) and then with brine solution. in Na 2 SO 4 The organic layer was dried on top, concentrated in vacuo to get the crude product, and silica gel chromatography (MeOH:DCM=1:20) gave leucine hexaalkyl ester (36%).
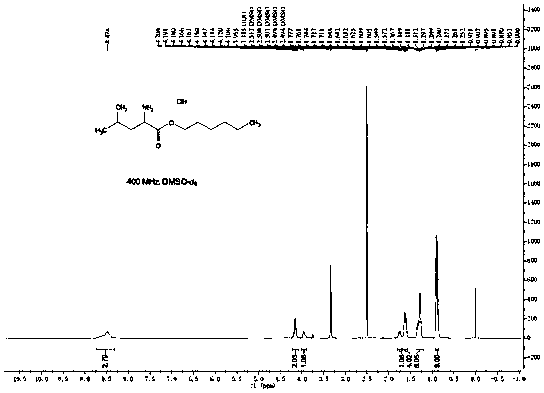
[0062] Figure 5 It is the NMR spectrum of leucine hexaalkyl ester, through Figure 5 It can be simply analyzed that the product is hexaalkyl leucine ester.
[0063] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com