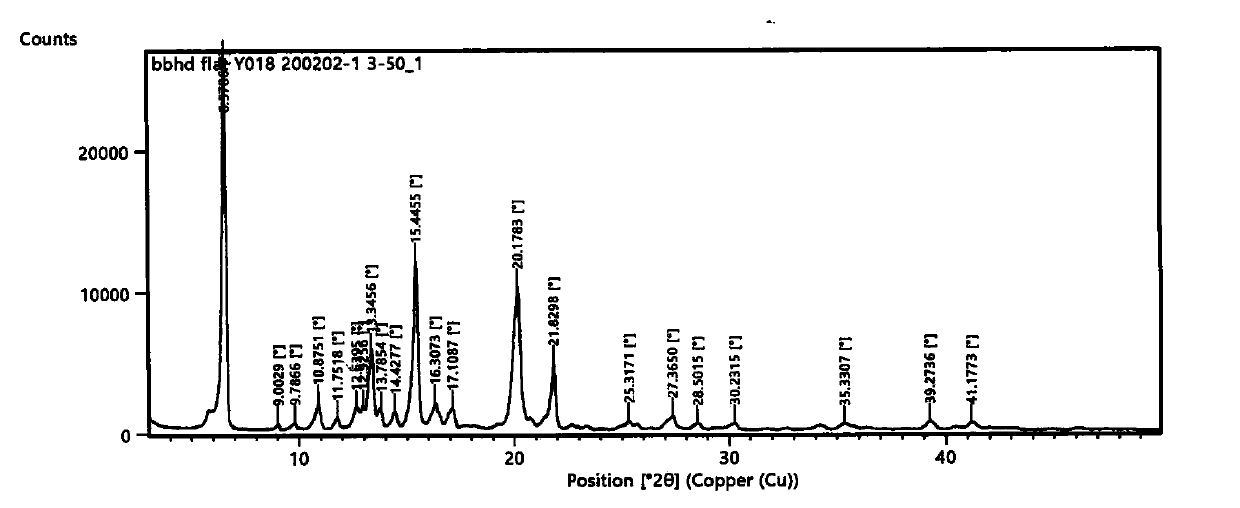
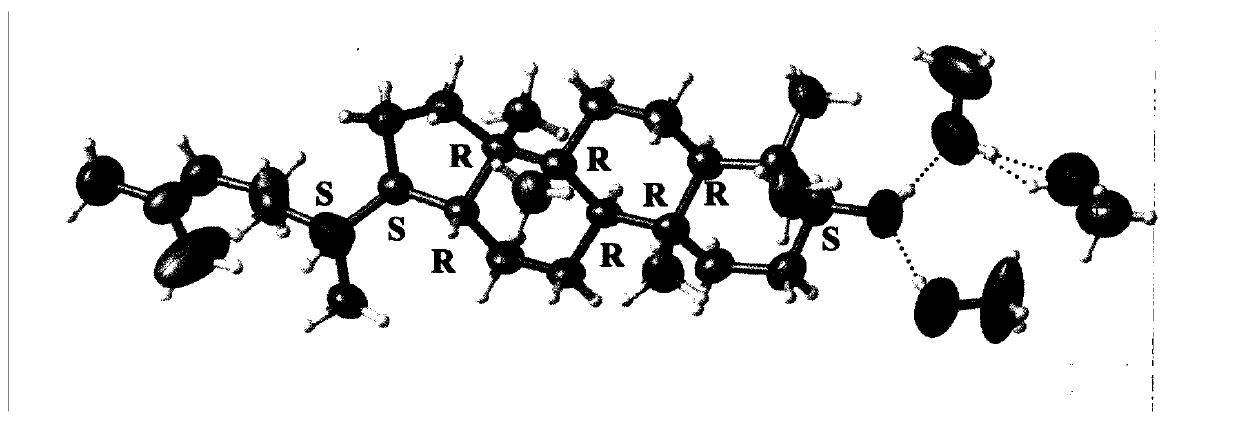
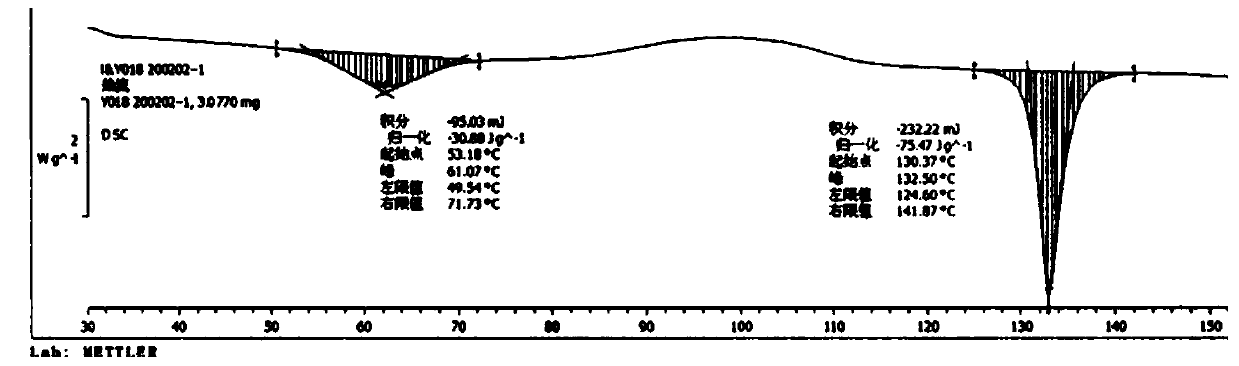
Dammarene diol trimethanol compound, crystal form A and preparation method thereof
A technology of damarene diol and alcoholate, applied in steroids, organic chemistry methods, organic chemistry and other directions, can solve the problems such as the research report on the crystalline form of damarene diol and its preparation method that have not been retrieved, and the like, Achieve the effect of good market prospect, high dissolution and easy dissociation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 Preparation of dammarene diol trimethanolate crystal form A
[0042] Add 1 g of dammarene diol into 12 ml of methanol, sonicate for 0.5 h, dissolve, and filter with suction. The sample liquid crystallizes naturally at room temperature for 36 h, filters, and vacuum-dries the filter cake to obtain the needle-shaped dammarene diol trimethanol crystal form. a.
Embodiment 2
[0043] Embodiment 2 Preparation of dammarene diol trimethanolate crystal form A
[0044] Add 1 g of dammarene diol into 5 ml of methanol, sonicate for 0.5 h, dissolve, and filter with suction. The sample liquid crystallizes naturally at room temperature for 12 h, filters, and vacuum-dries the filter cake to obtain the needle-shaped dammarene diol trimethanol crystal form. a.
Embodiment 3
[0045] The preparation of embodiment 3 dammarene diol trimethanol compound
[0046] Add 1 g of dammarene diol into 1 ml of methanol, sonicate for 0.5 h, dissolve, and filter with suction. The sample liquid crystallizes naturally at room temperature for 24 h, filters, and vacuum-dries the filter cake to obtain the needle-shaped dammarene diol trimethanol crystal form. a.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com