Improved diluent for cell-associated alphaherpesvirus vaccine
A herpes virus, herpes virus infection technology, applied in the field of vaccinology, can solve the problems of reducing the protective efficacy, not getting the full dose, loss of vaccine virus titer, etc., to achieve the effect of compensating for the loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Example 1: Overview of Stability Assays
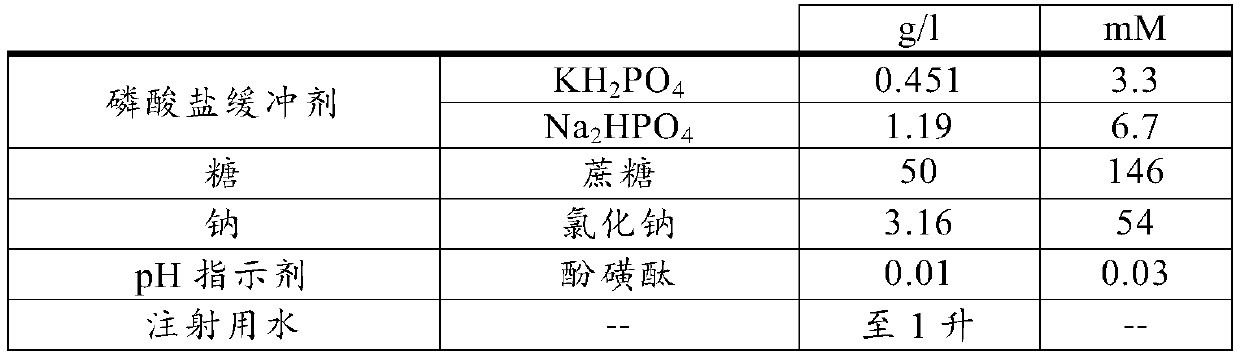
[0168] The basis of the diluent for use according to the invention is formed by phosphate buffer and sucrose.
[0169] Phosphate buffer was used at a concentration of 10 mM and set to a certain pH level by selecting the composition of the two phosphates. For example, for pH 7.3, the diluent contains: 0.324 mg / ml KH 2 PO4 and 1.356mg / ml Na 2 HPO 4 .2H 2 O.
[0170] Sucrose was used at a concentration of 50 mg / ml (ie, 150 mM), with some of the diluent samples tested having a higher sucrose content of 92.5 mg / ml (ie, 270 mM).
[0171] The concentration of phenolsulfonphthalein (Phenolsulfonphtalein) is 0.01 or 0.02 mg / ml.
[0172] In some diluent samples, with 1mM MgCl 2 Add the detected magnesium.
[0173] In some diluent samples, the CaCl tested was added at 0.133 mg / ml 2 .
[0174] In some diluent samples, the sodium citrate tested was added at 1 mM.
[0175] In some of the diluent samples tested, the following pepto...
Embodiment 2
[0180] Embodiment 2: the content detection of peptone in the diluent
[0181] In initial experiments, the effect of peptone content was examined. Recombinant HVP360-infected CEF samples were incubated for 4 hours at room temperature in diluent compositions containing varying amounts of NZ-amine. The diluent used in these experiments had a pH of 7.4 and contained magnesium and calcium ions. A series of samples were incubated in diluent plus 1% v / v NCS to simulate cell culture medium.
[0182] After incubation, samples were titrated (in triplicate) on CEF and the difference between the titers before incubation (t=0 hours) and after incubation (t=4 hours) was calculated as "delta". The standard deviation of titers is usually about 0.1. All titers are in 10 Log plaque forming units per ml.
[0183] Table 1: Effect of different contents of peptone on the in-use stability of recombinant HVT in CEF
[0184]
[0185] PB = phosphate buffer
[0186] From these results it is c...
Embodiment 3
[0187] Embodiment 3: the detection of a small amount of peptone
[0188] A follow-up experiment was performed to investigate the use of small amounts of peptone. This time the diluent did not contain magnesium or calcium, but different pH values were tested. Titrations were performed in duplicate.
[0189] Table 2: Effect of small amounts of peptone on in-use stability of recombinant HVT in CEF
[0190]
[0191] Several conclusions can be drawn from these results:
[0192] - The recombinant HVT construct was found to have the greatest loss (highest delta value after 4 hr at 25°C) when maintained in standard commercial MDV diluent (with 14 mg / ml peptone)
[0193] - Minimal loss of complete medium was found
[0194] -Basic diluents of phosphate buffer and sucrose were effective in reducing titer loss of recombinant HVT in CEF for up to 4 hours at room temperature
[0195] - No need for magnesium and calcium
[0196] - pH 7.4 diluents are more effective than pH 7.2 d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com