RET kinase inhibitor intermediates and preparation method thereof
A kinase inhibitor and preparation process technology, applied in the direction of organic chemistry, can solve the problems of high cost and poor stability of intermediates, and achieve the effects of improving production efficiency, simple waste liquid treatment, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
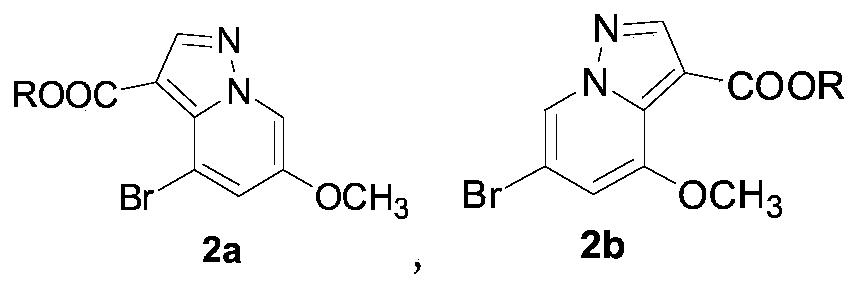
[0034] The synthesis of embodiment 1 compound 2a and 2b
[0035] Weigh 60g (322.5mmol) of 3-bromo-5-methoxypyridine, 180mL of water, and 36.5g (322.5mmol) of sulfamic acid into a 500mL flask, rise to 90°C for 30min, cool to room temperature, add Potassium carbonate 44.6g (322.5mmol) and absolute ethanol 130mL, ethyl propiolate 25.3g (257.9mmol) was added dropwise at room temperature, reacted overnight, filtered, washed with water, dried, red solid (2a and 2b mixture) 62.5g, Yield 81.0%.
Embodiment 2
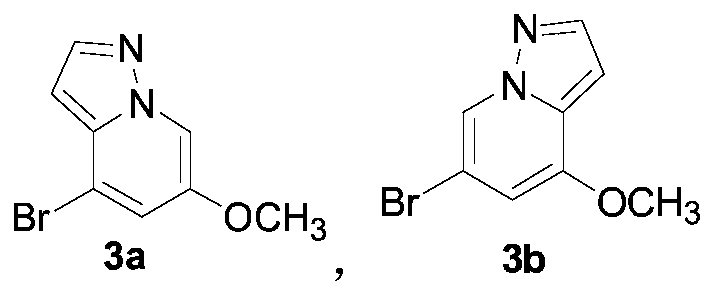
[0036] The synthesis of embodiment 2 compound 3a and 3b
[0037] Weigh 50.0g (167.2mmol) of the mixture of 2a and 2b and 350mL of 48% HBr aqueous solution, add them to a 500mL single-necked bottle, raise the temperature to 80°C for 2.5h, cool down in an ice bath and stir, and add potassium carbonate to the mother liquor until there is no Bubbles were generated, filtered, washed with water, and separated by column chromatography to obtain 11.3g of 3b and 15.2g of 3a. The total yield of 3a and 3b was 96.8%, and the ratio of 3a and 3b was 1:2.4.
[0038] 3a hydrogen spectrum data: 1 H NMR (400MHz, CDCl 3 δppm): δ3.82(s, 3H, OCH 3 ), 6.57 (d, J = 1.9Hz, 1H, Ar-H), 7.18 (d, J = 1.8Hz, 1H, Ar-H), 7.87 (d, J = 2.2Hz, 1H, Ar-H), 8.04 (d, J=1.8Hz, 1H, Ar-H); 3b hydrogen spectrum data: 1 H NMR (400MHz, CDCl 2 δppm): δ 3.82 (s, 3H, OCH 3 ), 6.57(d, J=1.9Hz, 1H, Ar-H), 7.18(d, J=1.8Hz, 1H, Ar-H), 7.87(d, J=2.2Hz, 1H, Ar-H), 8.04 (m, 1H, Ar-H).
Embodiment 3
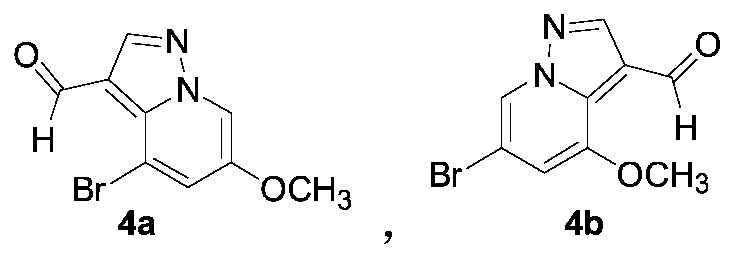
[0039] The synthesis of embodiment 3 compound 4a
[0040]Weigh 10.3g (45.4mmol) of 3a and 154.5mL of DMF, add them to a 250mL two-necked bottle respectively, cool down to 0°C, add 20.9g (136.1mmol) of phosphorus oxychloride dropwise, transfer to a 25°C water bath after adding React for 3 hours; pour the reaction liquid into 500 mL of ice water, add sodium hydroxide until the pH of the solution is 8.0-9.0, filter, wash with water, and dry to obtain 11.3 g of off-white solid 4a with a yield of 98.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com