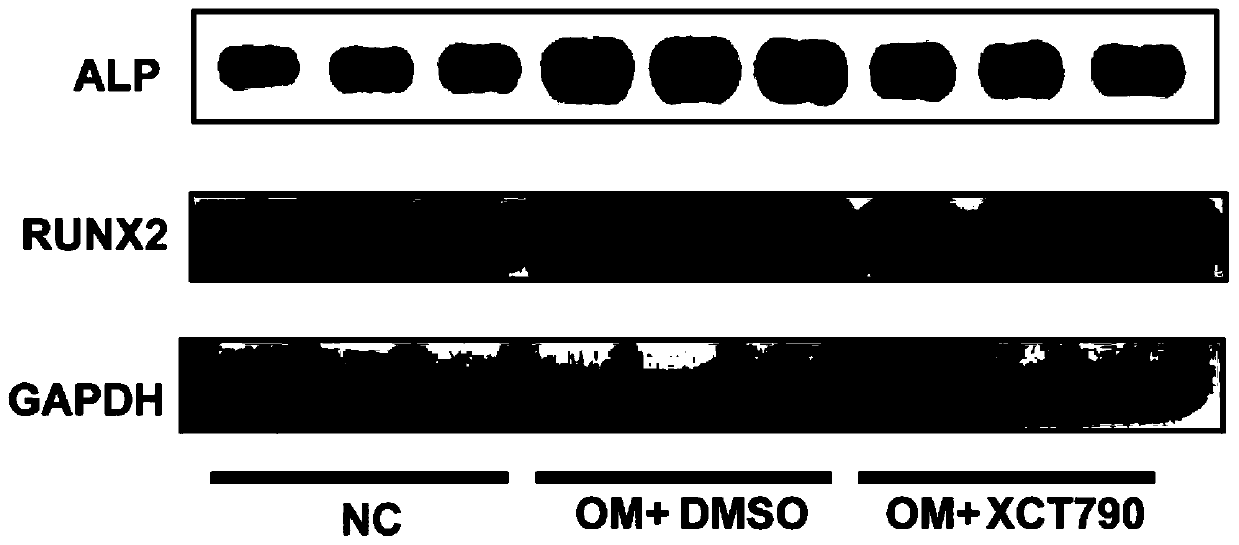
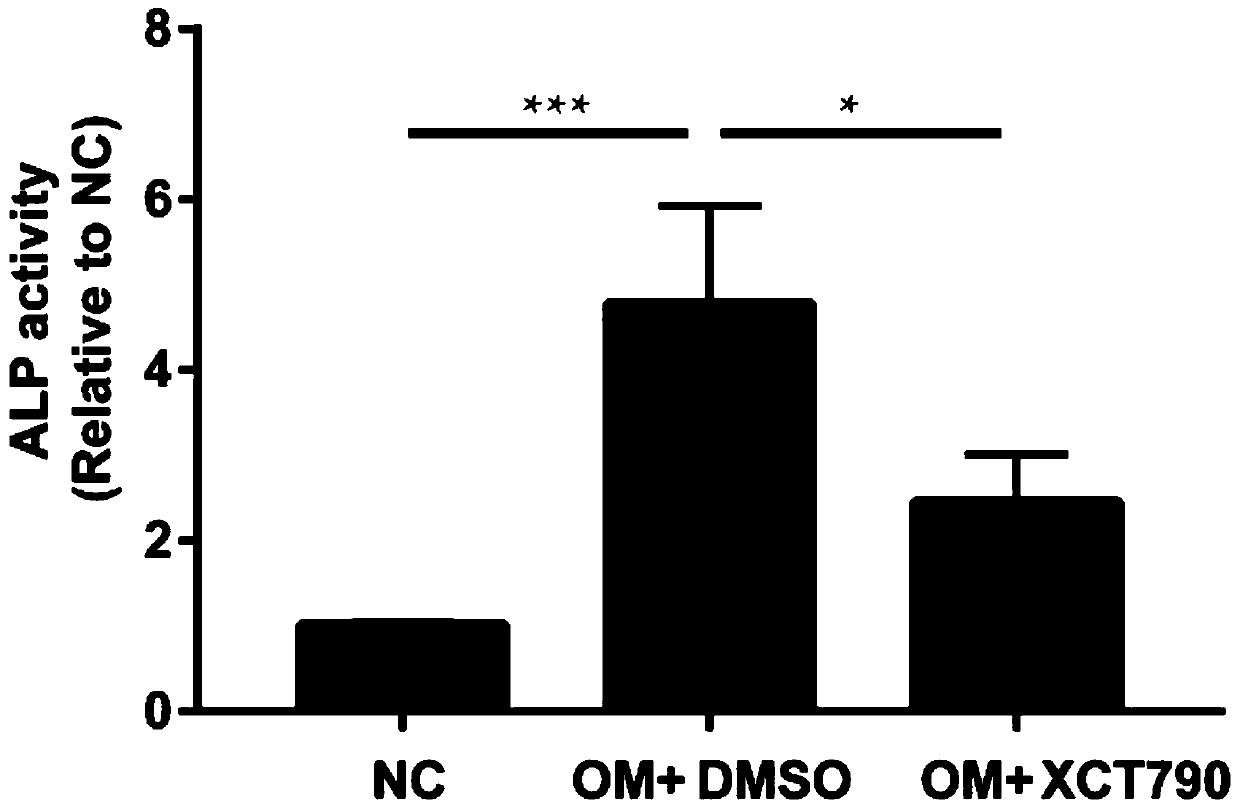
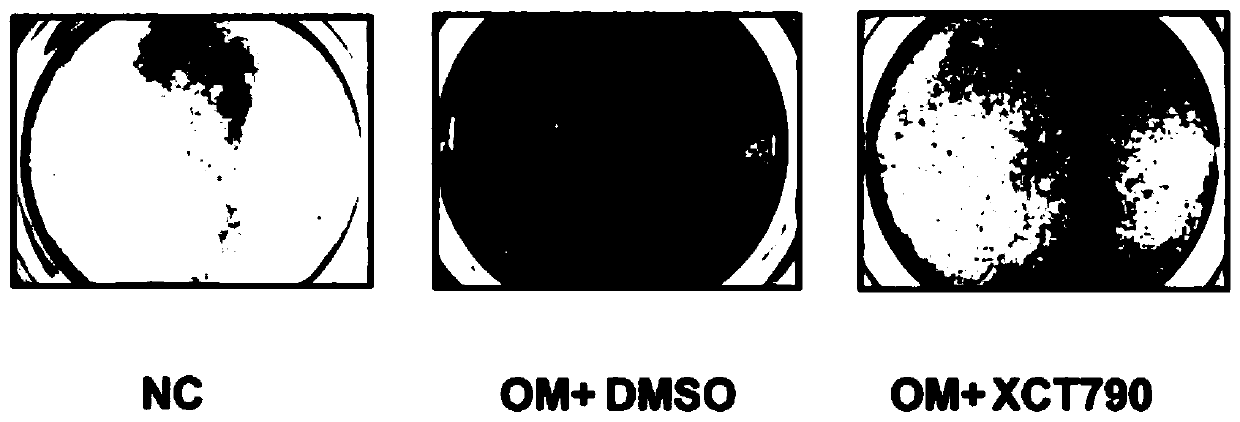
Application of XCT790 in preparation of medicine for treating calcified aortic valve diseases
A technology of XCT790, 1.XCT790, applied in the application field of XCT790 in the preparation of drugs for the treatment of calcific aortic valve disease, to achieve the effect of improving osteogenic differentiation in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Take the aortic valve tissue from the heart transplant recipient, store it in sterile PBS, and quickly transport it back to the laboratory with an ice box. On the primary cell laboratory, wash the valve with pre-cooled sterile PBS to remove residual Blood clot until the valve leaflets are white, the whole process should be strictly aseptic and the time between valve isolation and cell culture should be shortened as much as possible; the cleaned valve tissue should be placed in a solution containing penicillin (1000U / ml) and streptomycin ( 1000U / ml) in sterile PBS for 10-15 minutes.
[0025] 2. Weigh 100mg type II collagenase powder (purchased from Sigma-Aldrich, USA), suspend and dissolve in 50ml sterile PBS buffer, prepare 2% type II collagenase digestion solution, and then filter with 0.22um Net filter to remove bacteria in the digestive enzyme working solution and prevent cell contamination.
[0026] 3. Put the valve tissue into a 15ml sterile centrifuge tube, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com