Compound for treating acute lung injury as well as preparation method and application thereof
A compound and hydrophobic drug technology, applied in biochemical equipment and methods, medical preparations of non-active ingredients, non-active ingredients of polymer compounds, etc., can solve low drug loading, systemic side effects, and lung retention Short time and other issues, to achieve the effect of prolonging the residence time, improving targeted delivery, and protecting from degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1: Indomethacin drug crystal
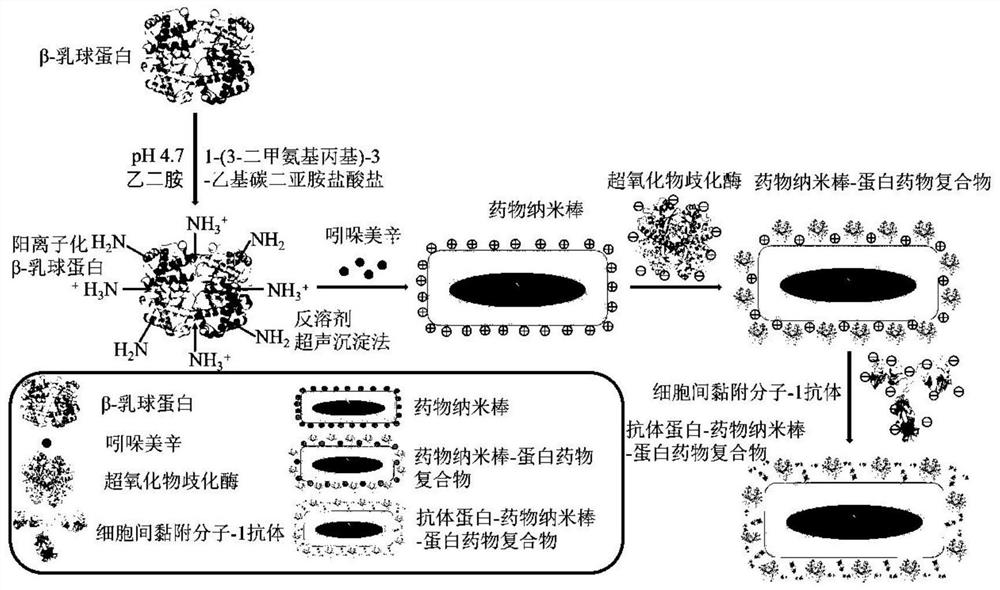
[0078] (1) The preparation flow chart is as follows figure 1 As shown, the formulation optimization process of indomethacin pharmaceutical crystals is as follows:
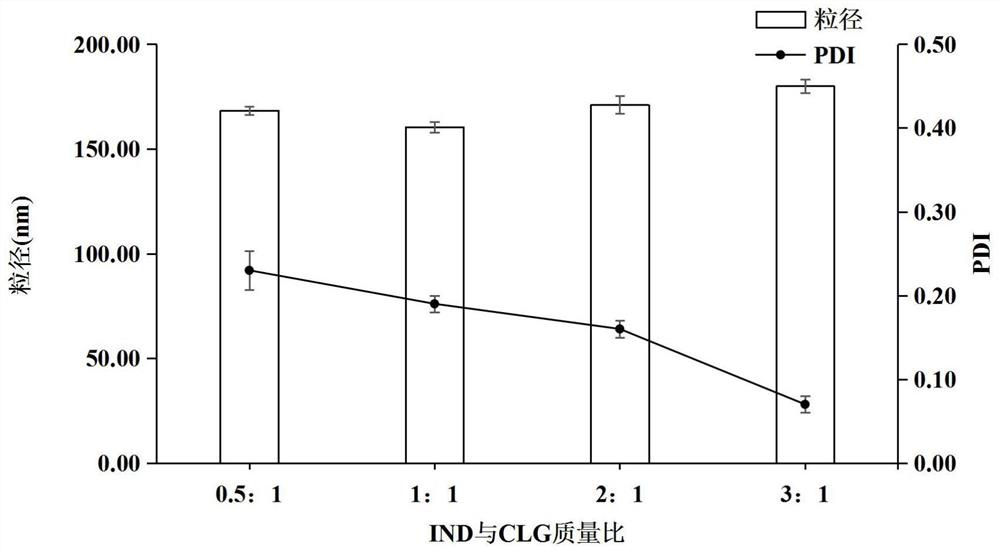
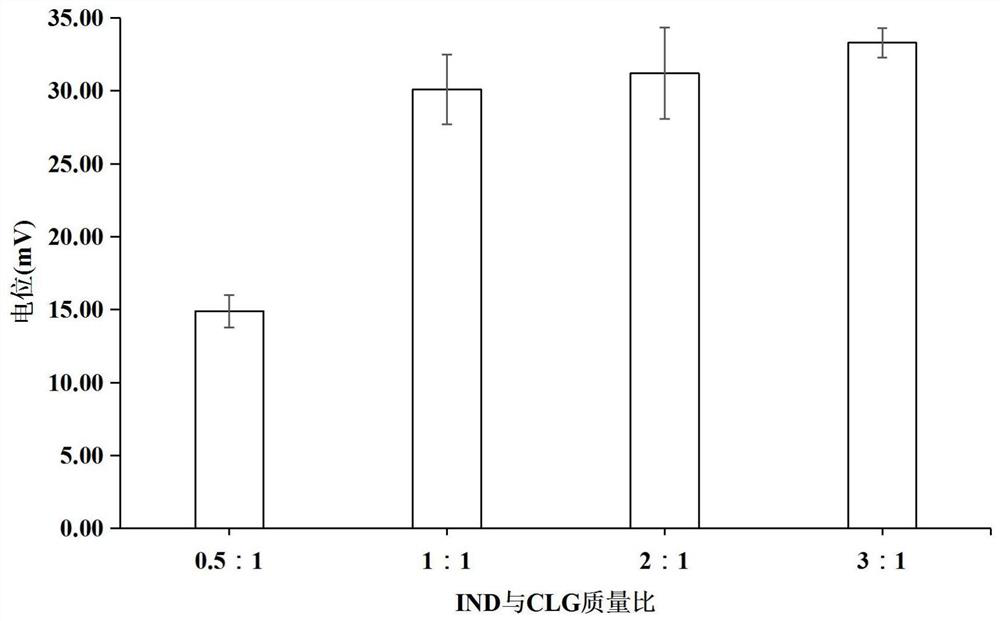
[0079] Weigh different indomethacin (5mg, 10mg, 20mg, 30mg) raw materials and dissolve them in 0.2ml acetone as the organic phase; weigh 10mg of cationized β-lactoglobulin and dissolve them in 10ml distilled water as the water phase; Both the organic phase and the aqueous phase were pre-cooled at 4°C; under ice bath conditions, the organic phase was added dropwise to the aqueous phase under the stirring condition of 1500r / min, and the probe was ultrasonicated immediately, and the ultrasonic condition was ultrasonic The power is 360W, the ultrasonic mode is on for 3s and off for 5s, and the total ultrasonic time is 15min. The residual organic solvent acetone is removed by vacuum evaporation to obtain indomethacin drug crystals.
[0080] The particle size and potenti...
Embodiment 2
[0086] Example 2: Indomethacin drug crystal-superoxide dismutase complex
[0087] (1) The formulation optimization process of indomethacin drug crystal-superoxide dismutase complex is as follows:
[0088] The nanorods were selected as indomethacin drug crystals in Example 1 (2), and the antioxidant protein was selected as superoxide dismutase.
[0089] Weigh an appropriate amount of superoxide dismutase, dissolve it with distilled water and dilute it into solutions of different concentrations, so that the mass ratio of cationized β-lactoglobulin to superoxide dismutase is 1.0, 2.0, 4.0, 16.0, 32.0 and 64.0, The indomethacin drug crystals in Example 1 (2) were added dropwise to an equal volume under vortexing conditions, and the indomethacin drug crystals-superoxide dismutase complex was obtained after standing at room temperature for 30 min.
[0090] The particle size and potential results of the prepared indomethacin drug crystal-superoxide dismutase complex are as follows: ...
Embodiment 3
[0094] Example 3: Verification of the formation of indomethacin drug crystal-superoxide dismutase complex by native polyacrylamide gel electrophoresis
[0095] The indomethacin drug crystal-superoxide dismutase complexes with the mass ratios of cationized β-lactoglobulin and superoxide dismutase of 1.0, 2.0, 4.0, 16.0, 32.0 and 64.0 were prepared respectively. Dismutase was used as a control, and was analyzed by non-denaturing polyacrylamide gel electrophoresis. The specific method is as follows: respectively preparing a 3% acrylamide / diacrylamide stacking gel (0.25M Tris-HCl, pH 6.8) and a 12.7% acrylamide / diacrylamide separating gel (0.75M Tris-HCl, pH 8.8), The loading amount of superoxide dismutase in each well was 1 μg. After loading, electrophoresis was carried out at a current of 20 mA. When the band reached the separation gel, the current was increased to 30 mA for electrophoresis separation for 1 h. After electrophoresis, the gel was transferred to Coomassie brillian...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com