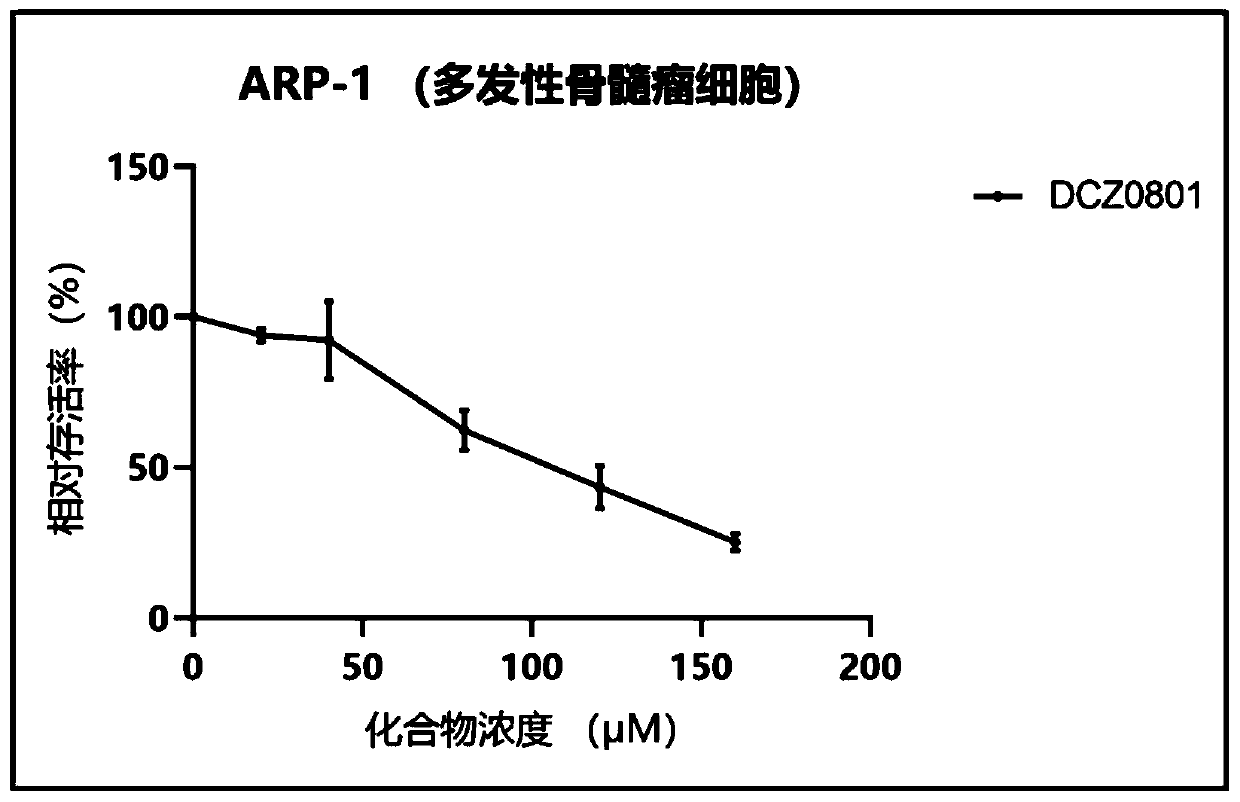
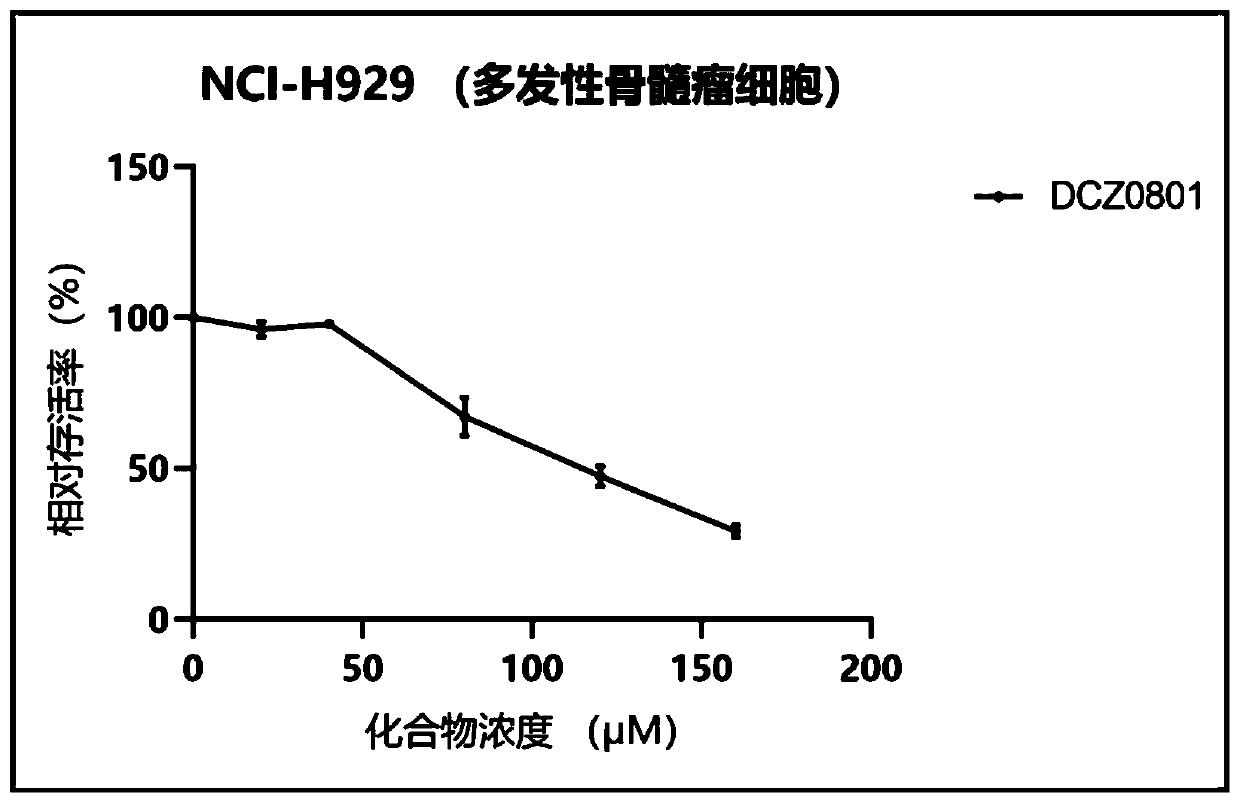
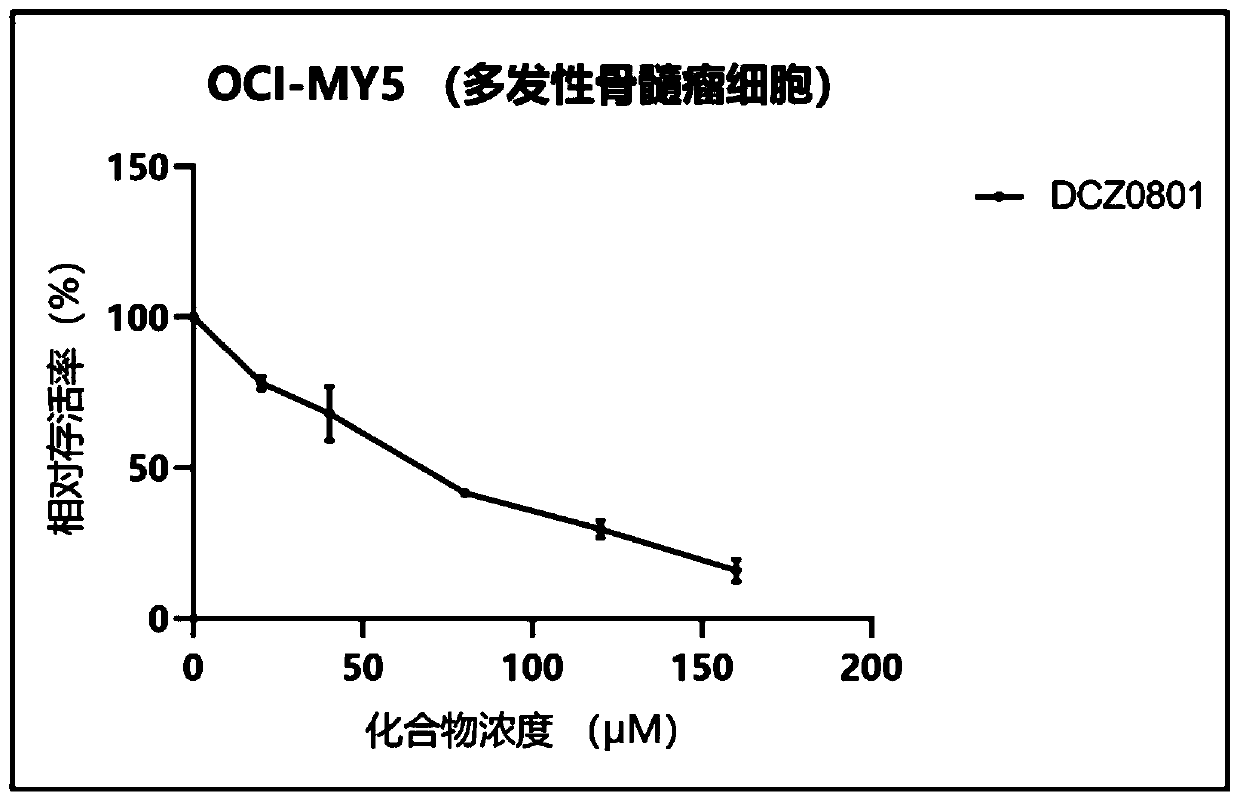
Osalmid and pterostilbene based cyclic coupling molecule DCZ0801 compound and preparation method and application thereof
A technology for coupling compounds, salivamol, which is applied in the field of ring-forming molecular coupling compounds and their pharmaceutical compositions, can solve problems such as limiting drug applications and lowering blood cholesterol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0045]
[0046] Phosphorus oxychloride (0.50mL, 5.5mmol) was dissolved in dichloromethane (60mL), under nitrogen protection, cooling in an ice bath, compound 1 (1.28g, 5.0mmol) and triethylamine (0.85mL, 6.0mmol) were added dropwise dichloromethane (15mL) solution, stirred for 2 hours, and took a sample to monitor that the reaction was complete. The reaction solution was cooled in an ice bath again, and then compound 2-1 (1.145 g, 5.0 mmol) and triethylamine (1.70 mL, 12.0 mmol) in acetone (10 mL) were added, stirred and reacted for 10 hours, and samples were taken to monitor the completion of the reaction. Dilute with water, extract the reaction liquid with dichloromethane, separate the organic phase, wash with water, wash with saturated sodium chloride, dry over anhydrous sodium sulfate, evaporate to dryness under reduced pressure, and the residue is separated by silica gel column (PE / EA(v:v) = 1:1;CH 2 Cl 2 / MeOH (v:v)=20:1), the white solid product DCZ0801 was obtaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com