Compound and application thereof in resistance to arenavirus infection
A compound, C1-C5 technology, used in antiviral agents, organic chemistry, etc., can solve the problems of high mortality, no vaccine, serious clinical manifestations, etc., and achieve broad-spectrum inhibitory activity and strong inhibitory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
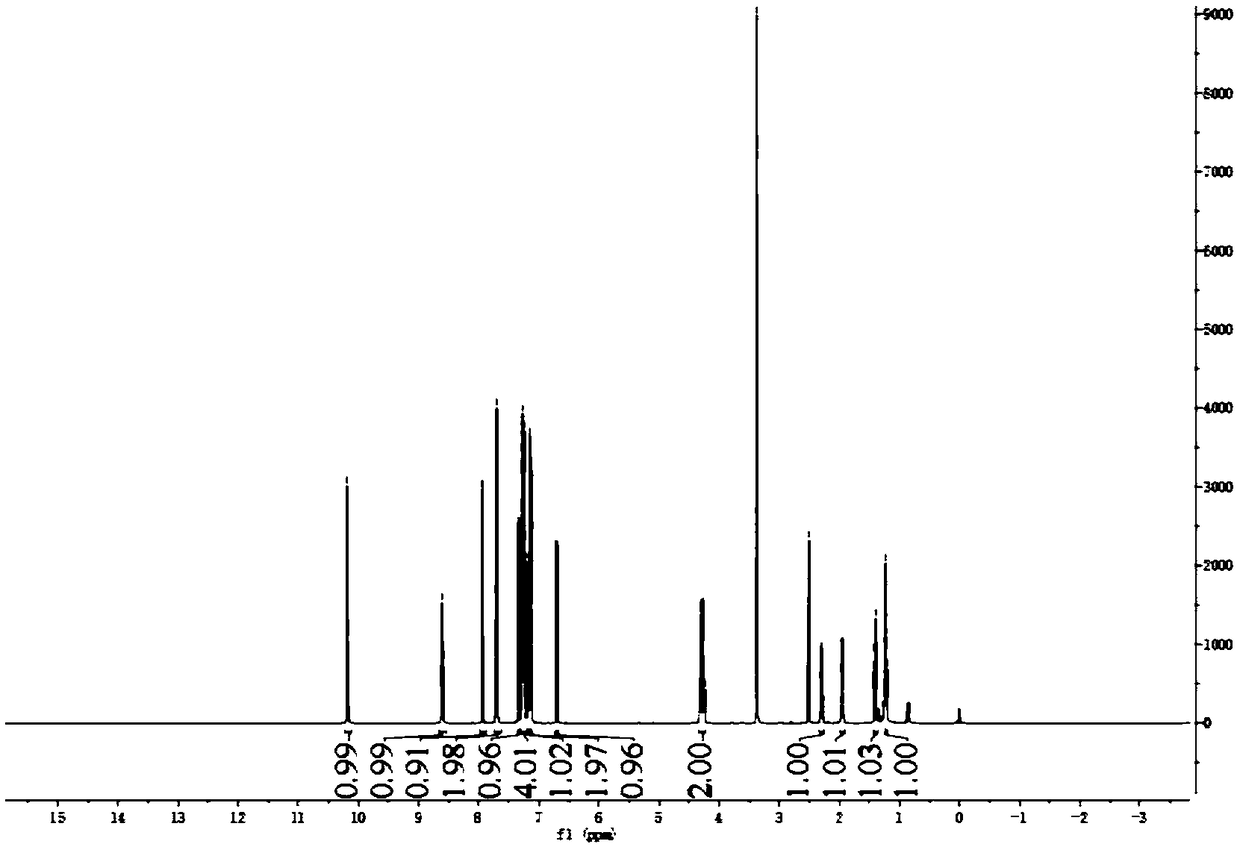
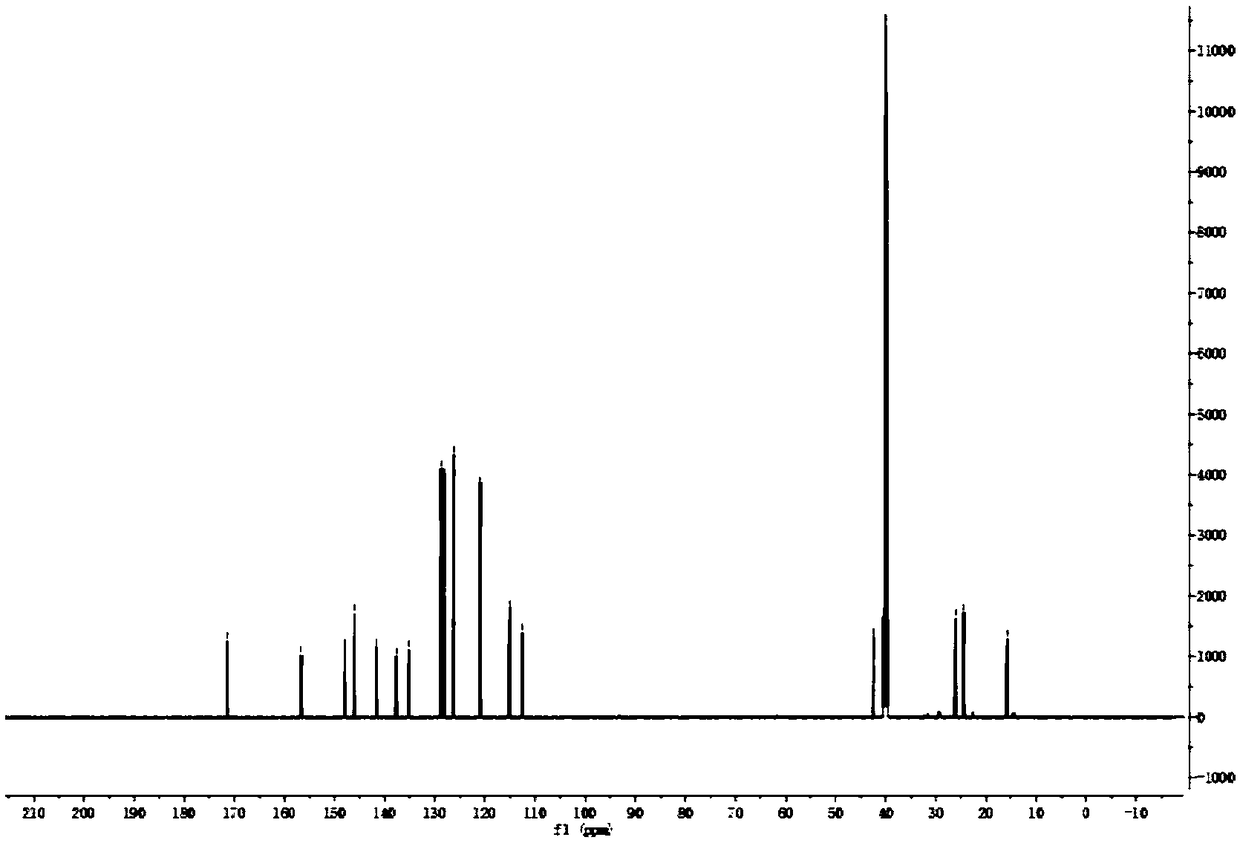
Examples
Embodiment 1
[0036]
[0037] The specific process is:
[0038] (1) Take a 50ml round bottom flask and add 15mL of ethanol and 1g of compound A in turn, slowly add 0.36g of NaBH to the system 4 (sodium borohydride), the system is white and turbid, and the reaction at room temperature ends after 3 hours. Use 1mol / L HCl (hydrochloric acid) to adjust the pH to 5, then remove the solvent, extract twice with water and ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, and concentrate in vacuo to obtain 1 g of a white solid, namely compound B, with a yield of 99%.
[0039] (2) Take a 50mL round bottom flask and add 3ml of dichloromethane, 0.15g of compound B, 0.16g of compound C and 0.62g of EDCI (1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride salt), 0.4g DMAP (4-dimethylaminopyridine), the system is yellow and clear, and the reaction is completed after stirring at room temperature for 5h, and then the system is respectively treated with 1mol / L HCl and ...
Embodiment 2
[0041]
[0042] The specific process is:
[0043] (1) Take a 100mL round-bottom flask and add 10mL of dichloromethane, 0.5g of p-nitrobenzyl bromide, and 0.64g of phthalamide potassium salt in turn, and stir at room temperature to react. The system is white and turbid, and then slowly turns purple. After 2 hours of reaction at room temperature, the system was extracted twice with dichloromethane and water, the organic phases were combined and dried, and 0.65 g of a yellow solid, namely compound E, was obtained by rotary evaporation, with a yield of 100%.
[0044] (2) Take a 50mL round bottom flask and add 5mL ethanol and 0.27g compound E successively. The system is yellow and turbid. After adding 3.5mL80% hydrazine hydrate, the system becomes red and turbid. After 2h reaction at room temperature, the system is concentrated in vacuum and used Ethyl acetate and water were extracted twice, the organic phases were combined and dried over anhydrous sodium sulfate, and concentrat...
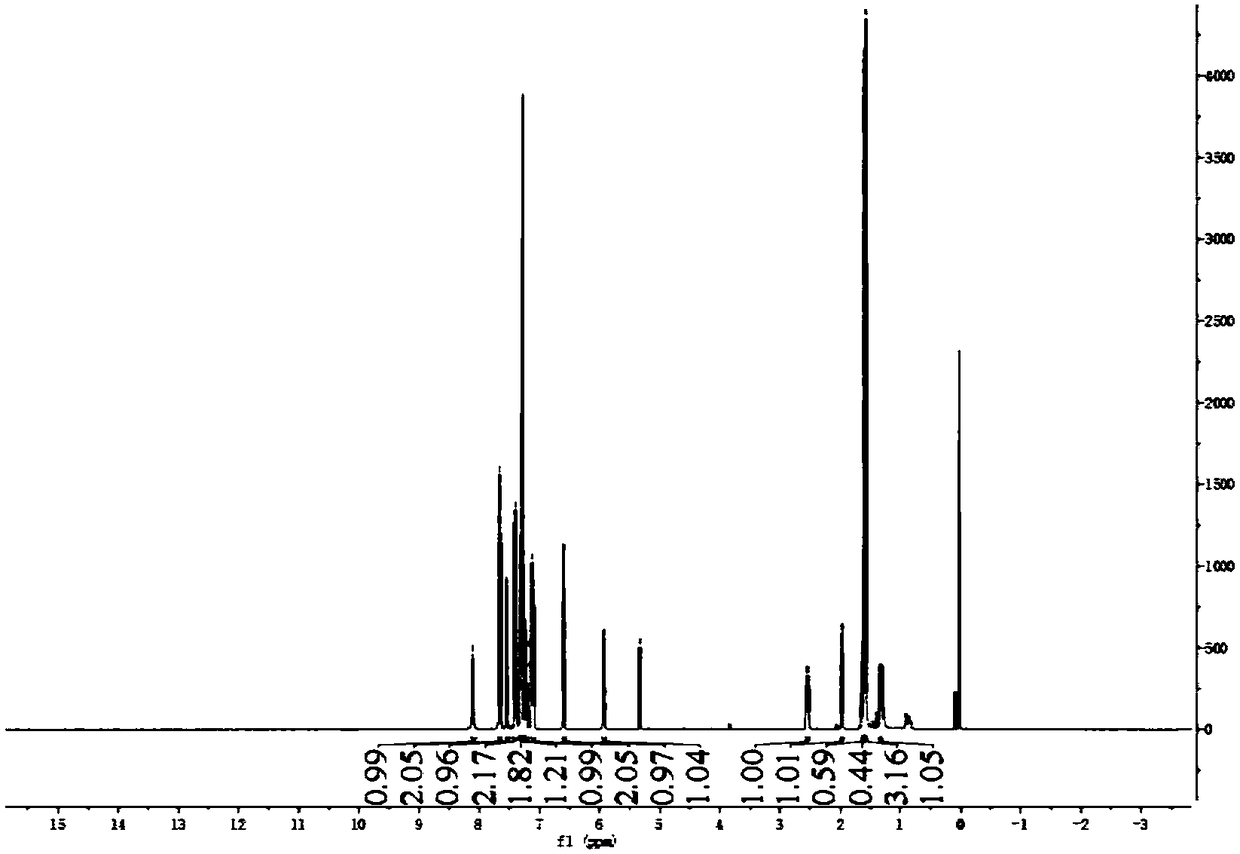
Embodiment 3
[0049]
[0050] The specific process is:
[0051] Take a 50mL flask and add 3mL of dichloromethane, 0.1g of compound I, 0.12g of compound B, 0.41g of EDCI, 0.26g of DMAP, and stir the reaction system at room temperature. / L HCl and saturated NaHCO 3 After washing, the organic phase was collected and dried with anhydrous sodium sulfate, and then the solvent was removed by rotary evaporation, and 0.14 g of a yellow liquid was obtained by purification with a silica gel column, namely compound J (ie, compound 9 of the above compound), and the reaction yield was 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com