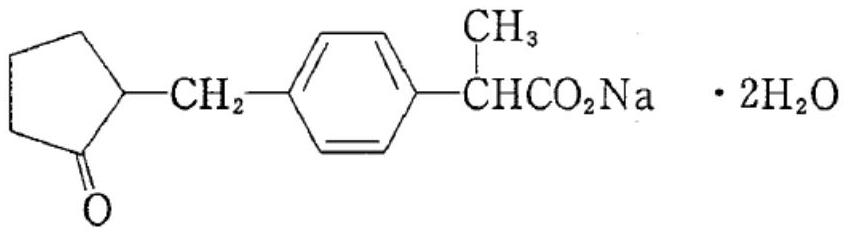
Loxoprofen sodium gel and preparation method thereof
A technology of loxoprofen sodium and gel, which is applied in the direction of medical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of high price and scarcity of loxoprofen sodium gel products and other issues to achieve the effects of reducing material loss, process optimization, and reducing preparation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
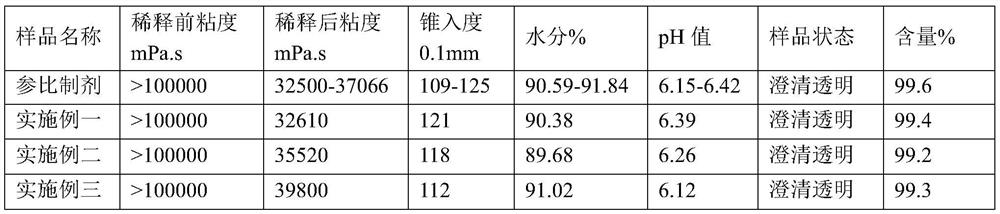
[0013] This embodiment provides a kind of loxoprofen sodium gel, and its raw material comprises the following components by weight percentage:
[0014] components Dosage g percentage% loxoprofen sodium 0.286 1.144 carbomer 0.15 0.6 hypromellose 0.3 1.2 1.3 Butanediol 1.25 5 Triethanolamine 0.3 1.2 ethanol 2 8 water 20.714 82.856
[0015] The preparation method of above-mentioned loxoprofen sodium gel is as follows:
[0016] (1) wetting carbomer and hypromellose with 1.3-butanediol and ethanol;
[0017] (2) Add the water of 60% recipe quantity in step (1), stir until even while adding;
[0018] (3) Add an aqueous solution of triethanolamine prepared by triethanolamine and 10% prescription water into step (2), and keep stirring until uniform;
[0019] (4) Add the aqueous solution of loxoprofen sodium prepared by loxoprofen sodium and 10% prescription water to step (3), adjust the pH value to 6.2, and keep stirri...
Embodiment approach 2
[0022] This embodiment provides a kind of loxoprofen sodium gel, and its raw material comprises the following components by weight percentage:
[0023] Element Dosage g percentage% loxoprofen sodium 0.286 1.144 carbomer 0.25 1 hypromellose 0.2 0.8 1.3 Butanediol 1.25 5 Triethanolamine 0.3 1.2 ethanol 2 8 water 20.714 82.856
[0024] The preparation method of the above-mentioned loxoprofen sodium gel is roughly the same as Embodiment 1, except that in step (4), the aqueous solution of loxoprofen sodium is added, and after adjusting the pH value to 6.25, the stirring is continued until uniform. In addition, the preparation method of this embodiment is completely the same as Embodiment 1, and will not be repeated here.
Embodiment approach 3
[0026] This embodiment provides a kind of loxoprofen sodium gel, and its raw material comprises the following components by weight percentage:
[0027] Element Dosage g percentage% loxoprofen sodium 0.286 1.144 carbomer 0.35 1.4 hypromellose 0.1 0.4 1.3 Butanediol 1.25 5 Triethanolamine 0.3 1.2 ethanol 2 8 water 20.714 82.856
[0028] The preparation method of the above-mentioned loxoprofen sodium gel is roughly the same as Embodiment 1, except that in step (4), the aqueous solution of loxoprofen sodium is added, and after adjusting the pH value to 6.27, the stirring is continued until uniform. In addition, the preparation method of this embodiment is completely the same as Embodiment 1, and will not be repeated here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com