Preparation method of alpha-acetyl-gamma-butyrolactone
A technology of butyrolactone and acetyl, which is applied in the field of preparation of α-acetyl-γ-butyrolactone, can solve the problems of low yield, less than 85%, achieve simple separation system, reduce hydrolysis and side reactions little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
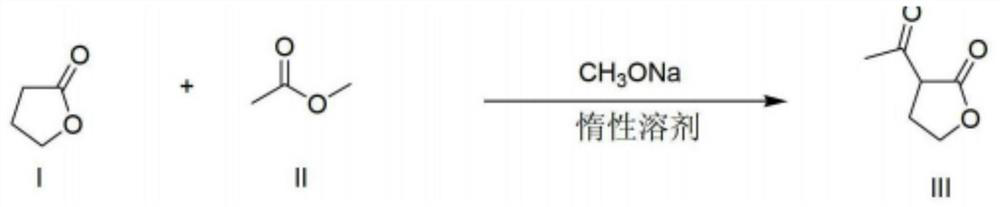
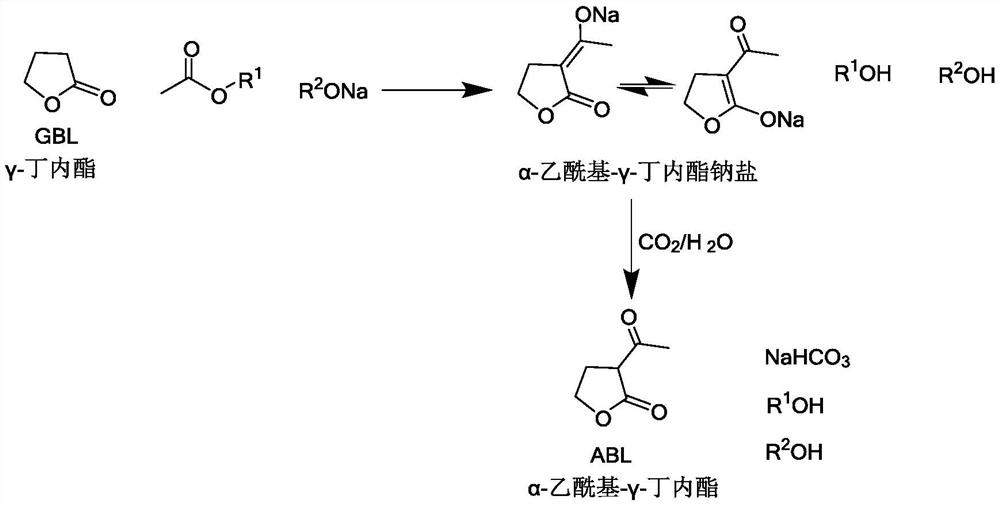
[0022] The present invention provides a kind of preparation method of α-acetyl-γ-butyrolactone, the method comprises the following steps:
[0023] (1) Make γ-butyrolactone, CH 3 COOR 1 and R 2 ONa undergoes acylation reaction to obtain a material containing α-acetyl-γ-butyrolactone sodium salt, wherein R 1 and R 2 Each is independently a C1-C4 alkyl group;
[0024] (2) Under the condition that water exists, make the material containing α-acetyl-γ-butyrolactone sodium salt and CO 2 A neutralization reaction occurs on gas contact.
[0025] According to the method of the present invention, preferably, in step (1), R 1 and R 2 Each independently preferably is a C1-C2 alkyl group such as methyl, ethyl. R 1 and R 2 Can be the same or different.
[0026] According to the method of the present invention, in step (1), the acylation reaction is generally carried out under dry conditions, and the acylation reaction is preferably carried out in a reaction tank equipped with a r...
Embodiment 1
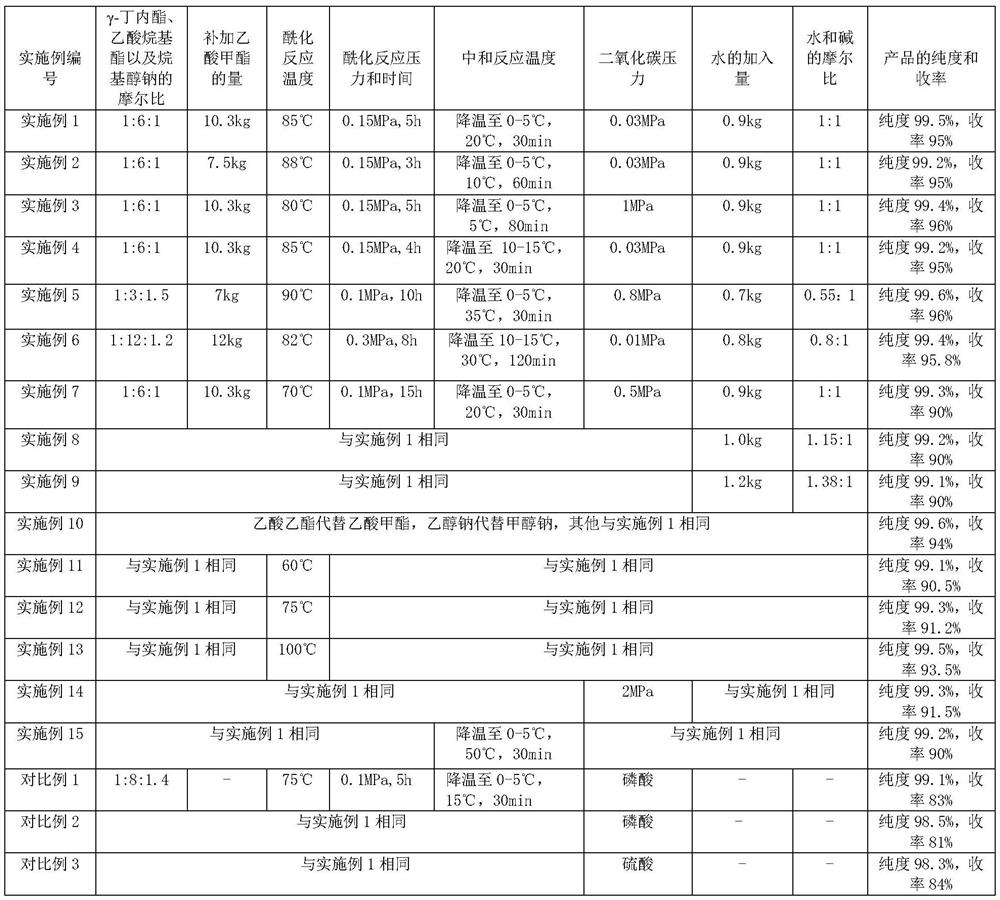
[0054] (1) Acylation reaction: the reaction is carried out in a dry 50L stainless steel reaction tank equipped with stirring, reflux and distillation devices. Turn on the stirring, replace the reaction tank with nitrogen, add 20.7kg of methyl acetate, raise the temperature in the reaction tank to 45°C, add 4kg of γ-butyrolactone and 2.6kg of sodium methylate in batches, and keep the system temperature at 43°C to between 48°C. Gradually raise the temperature of the reaction system to 55°C, slowly open the valve of the distillation device, control the reflux ratio to 4:1, collect about 20kg of the mixture of methanol and methyl acetate generated in the reaction, and use it for rectification, separation and recovery. At the same time, 10.3kg of methyl acetate was added slowly, the temperature was raised slowly, the pressure inside the tank was 0.15MPa, and the pressure was maintained at 85°C for 5h. About 1% of γ-butyrolactone remains in the reaction system monitored by gas chro...
Embodiment 2
[0059] (1) Acylation reaction: the reaction is carried out in a dry 50L stainless steel reaction tank equipped with stirring, reflux and distillation devices. Turn on the stirring, replace the reaction tank with nitrogen, add 20.7kg of methyl acetate, raise the temperature in the reaction tank to 45°C, add 4kg of γ-butyrolactone and 2.6kg of sodium methylate in batches, and keep the system temperature at 43°C to between 48°C. Gradually raise the temperature of the reaction system to 55°C, slowly open the valve of the distillation device, control the reflux ratio to 4:1, collect about 20kg of the mixture of methanol and methyl acetate generated in the reaction, and use it for rectification, separation and recovery. At the same time, 7.5 kg of methyl acetate was added slowly, the temperature was raised slowly, the pressure in the tank was 0.15 MPa, and the pressure was kept at 88° C. for 3 h. About 3% of γ-butyrolactone remains in the reaction system monitored by gas chromatogr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com