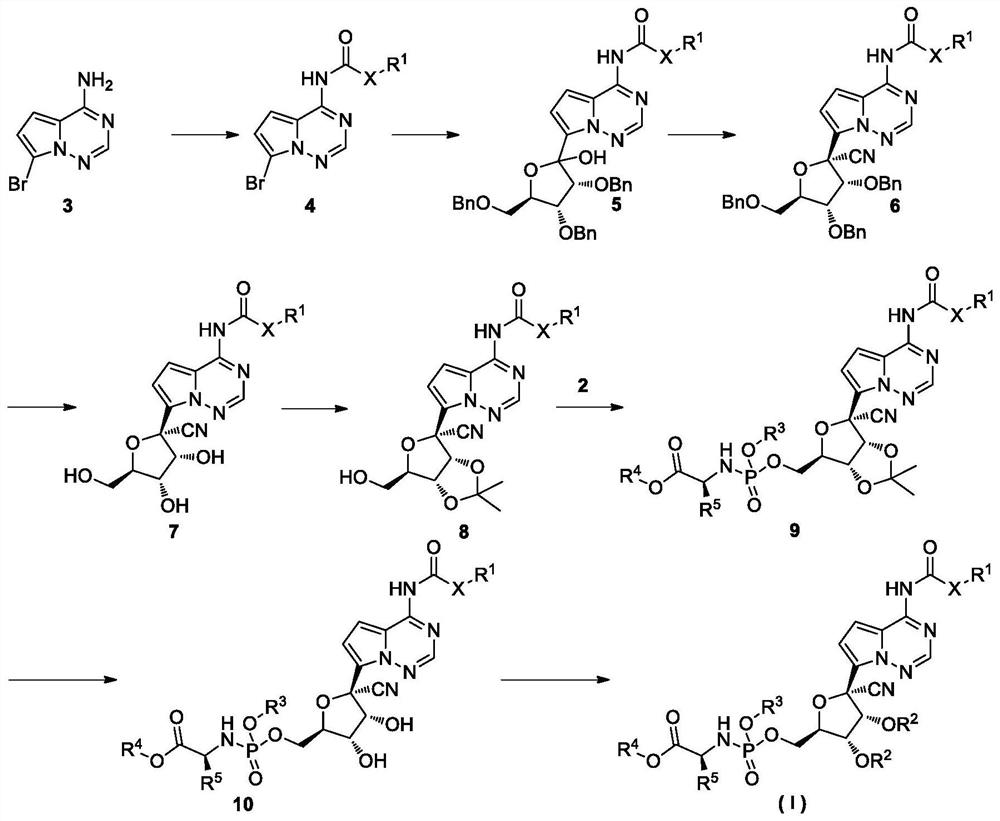
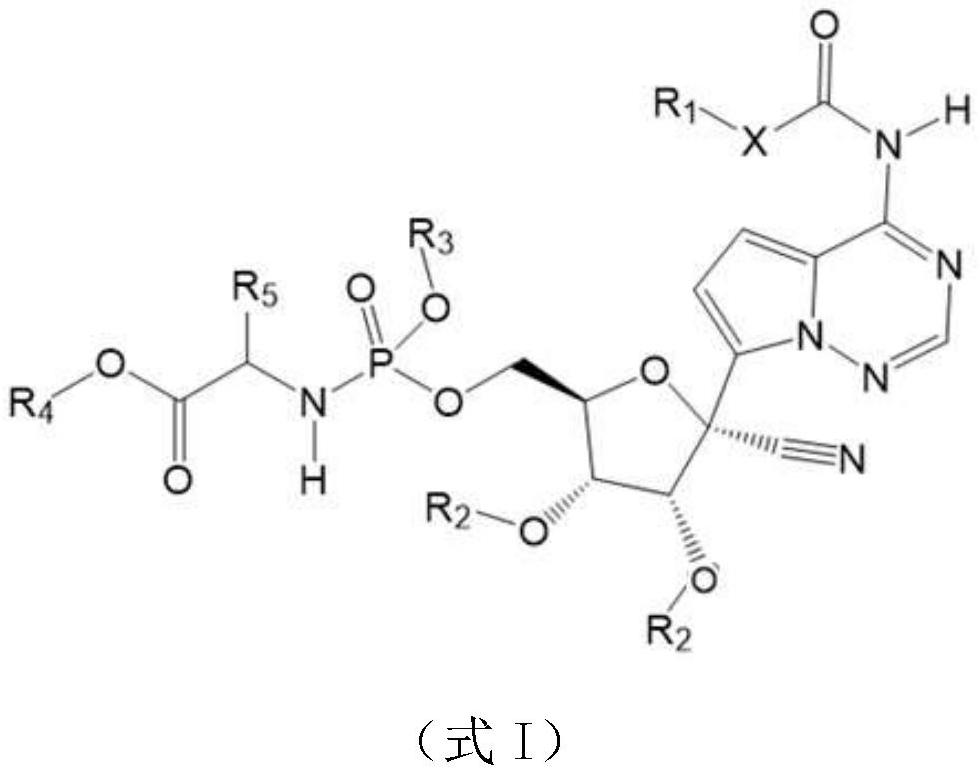
Prodrug of retegravir as well as preparation method and application of prodrug
A technology of compounds and general formulas, which is applied in the direction of pharmaceutical formulas, chemical instruments and methods, medical preparations containing active ingredients, etc., can solve problems such as high risks, side effects, and inconvenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
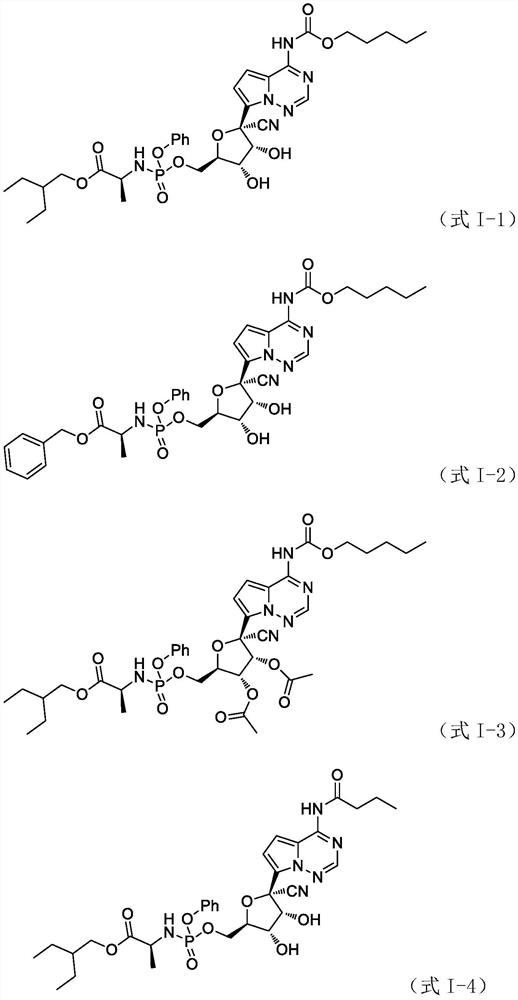
[0080] Example 1: (2S)-2-ethylbutyl 2-((((((2R,3S,4R,5R)-5-cyano-3,4-dihydroxy-5-(4-(((penta (Oxy)carbonyl)amino)pyrrolo[2,1-f][1,2,4]triazin-7-yl)tetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propionic acid Synthesis of esters (Formula I-1)
[0081]
[0082] 1) Synthesis of (2S)-2-ethylbutyl 2-(((4-nitrophenyl)(phenoxy)phosphoryl)amino)propionate
[0083]
[0084] Weigh alanine-2-ethylbutyl ester hydrochloride (21.0 g, 100 mmol, 1.0 eq.) into a 500 mL three-necked round bottom reaction flask under nitrogen protection, and add anhydrous dichloromethane (200 mL). The reaction solution was cooled to -70°C, phenyl dichlorophosphate (21.0 g, 100 mmol, 1.0 eq.) was added, and TEA (20.0 g, 200 mmol, 2.0 eq.) was slowly added dropwise, and the reaction was kept constant for 2 hours. TLC tracked until the raw material alanine-2-ethylbutyl ester hydrochloride was consumed, and 4-nitrophenol (14.0g, 100mmol, 1.0eq.) in dichloromethane (20mL) was added dropwise, at a constant tempe...
Embodiment 2
[0106] Example 2. (2S)-Benzyl 2-(((((2R,3S,4R,5R)-5-cyano-3,4-dihydroxy-5-(4-(((pentoxy) (Carbonyl)amino)pyrrolo[2,1-f][1,2,4]triazin-7-yl)tetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propionate Synthesis of (Formula I-2)
[0107]
[0108] Comprehensive literature preparation method, using 7-bromopyrrolo[1,2-f][1,2,4]triazine-4-amine as raw material, synthetically obtained white foamy solid (2S)-benzyl 2-(( (((2R,3S,4R,5R)-5-cyano-3,4-dihydroxy-5-(4-(((pentyloxy)carbonyl)amino)pyrrolo[2,1-f][ 1,2,4]Triazine-7-yl)tetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propionate.
[0109] 1 H NMR(CDCl 3 400MHz) δ9.67 (s, 1H), 7.47-7.18 (m, 10H), 5.89-5.72 (d, 2H), 5.34 (s, 2H), 4.53-3.65 (m, 8H), 1.62-0.90 (m ,12H).ESI-MS m / z:723.5[M+H]+.
Embodiment 3
[0110] Example 3, (2R, 3R, 4R, 5R)-2-cyano-5-((((((S)-1-(2-ethylbutoxy)-1-propionate-2-yl )Amino)(phenoxy)phosphoryl)oxy)methyl)-2-(4-(((pentyloxy)carbonyl)amino)pyrrolo[2,1-f][1,2,4] Synthesis of triazine-7-yl)tetrahydrofuran-3,4-diacetate (Formula I-3)
[0111]
[0112] Comprehensive literature preparation methods, with 7-bromopyrrolo[1,2-f][1,2,4]triazine-4-amine as raw material, a white foamy solid (2R, 3R, 4R, 5R) was synthesized by synthesis 2-cyano-5-((((((S)-1-(2-ethylbutoxy)-1-propionate-2-yl)amino)(phenoxy)phosphoryl)oxy) Methyl)-2-(4-(((pentyloxy)carbonyl)amino)pyrrolo[2,1-f][1,2,4]triazin-7-yl)tetrahydrofuran-3,4-di Acetate. 1 H NMR(CDCl 3 400MHz)δ9.67(s,1H),7.37-7.21(m,5H),5.89-5.72(d,2H),5.33-3.65(m,10H),2.21-0.98(m,29H).ESI-MS m / z:801.5[M+H]+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com