Quality control method for volatile components of Shenbao tablets
A quality control method and technology for volatile components, applied in the field of pharmacy, can solve the problems of no quantitative determination method and insufficient product quality evaluation, and achieve the effects of product stability, good accuracy and comprehensive product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1, the screening process of the content determination of trans-anethole in Shenbao tablets
[0062] 1.1 Instruments and materials
[0063] Chromatograph: Agilent 1100 high performance liquid chromatograph;
[0064] Chromatographic column: Agilent ZORBAX SB-C18 (4.6×150 mm, 5 μm);
[0065] Reagents: absolute ethanol, ethyl acetate, n-hexane, phosphoric acid, analytically pure; methanol, chromatographically pure;
[0066] Reference substance: trans-anethole, China National Institutes for Food and Drug Control, purity 99.6%, batch number: 111835-201804.
[0067] Sample: Jiangxi Huiren Pharmaceutical Co., Ltd., batch number: 1905288.
[0068] 1.2 Choice of method
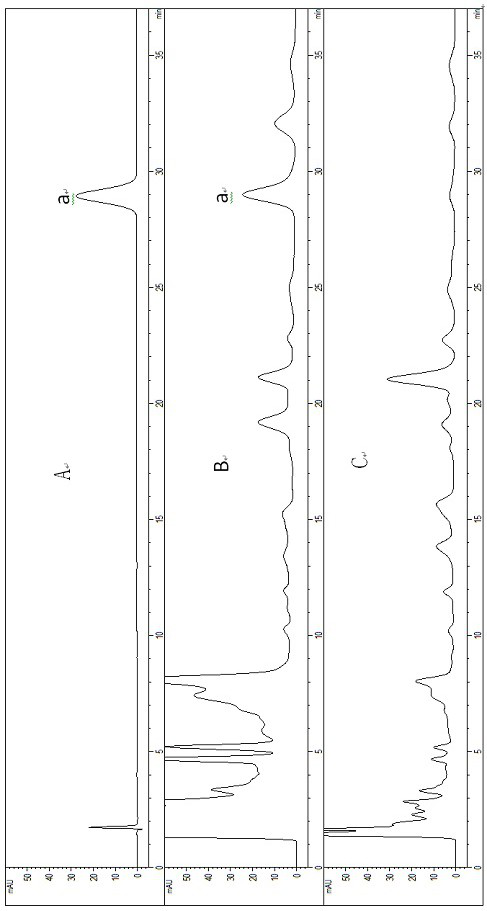
[0069] According to literature research and GC-MS research results of volatile components in Shenbao Tablets, trans-anethole from cumin and ligustilide from Ligusticum chuanxiong and Angelica sinensis were selected as index components for content determination.
[0070] Using GC chromatography, th...
Embodiment 2
[0132] The screening process of the content determination of ligustilide in embodiment 2, Shenbao tablets
[0133] 2.1 Instruments and materials
[0134] Chromatograph: gas chromatograph, Agilent 7890B; electronic thermostat electric heating mantle, model DZTW, power 0.5 KW.
[0135] Chromatographic column: Agilent HP-5, 30 m×0.32 mm×0.25 μm;
[0136] Reagents: absolute ethanol and xylene were analytically pure, Beijing Tongguang Fine Chemical Company; anhydrous sodium sulfate was analytically pure, Beijing Chemical Plant; water, prepared by Millipore clear-D ultrapure water integrated system; nitrogen, high-purity Nitrogen, 99.999% pure.
[0137] Reference substance: ligustilide, batch number MUST-19041005, purity 99.55%, Chengdu Master Biotechnology Co., Ltd.;
[0138] Sample: Shenbao Tablets, provided by Jiangxi Huiren Pharmaceutical Co., Ltd., batch number: 1905288.
[0139] 2.2 Choice of method
[0140] According to literature research and GC-MS research results of v...
Embodiment 3
[0195] Embodiment 3, the content assay method of trans-anethole
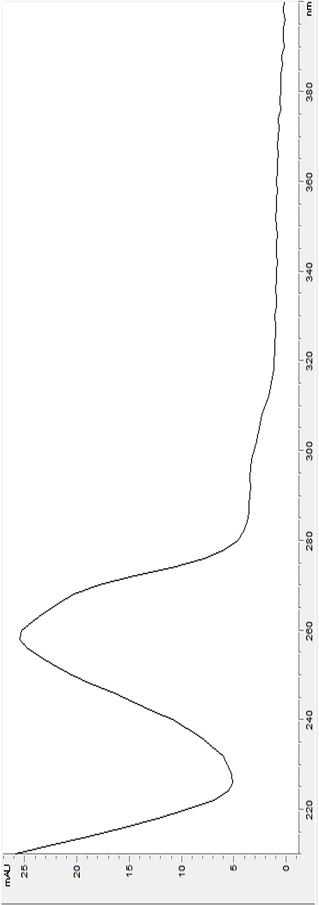
[0196] Chromatographic conditions and system suitability test Octadecylsilane bonded silica gel was used as filler; methanol-0.1% phosphoric acid water (55:45) was used as mobile phase; column temperature was 30°C; flow rate was 1.0ml / min; detection wavelength was 254 nm; the theoretical plate number should not be less than 5000 based on the trans-anethole peak,
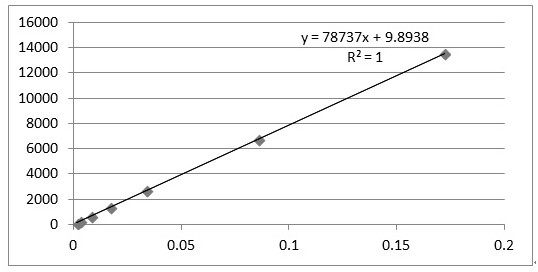
[0197] Preparation of reference substance solution Take an appropriate amount of trans-anethole reference substance, weigh it accurately, add absolute ethanol to make a solution containing 14 μg per 1 ml, to obtain,
[0198] Preparation of the test solution Take Shenbao tablet, pulverize it with a pulverizer, take about 2 g, accurately weigh it, put it in a stoppered Erlenmeyer flask, accurately add 20 ml of absolute ethanol, seal it tightly, weigh it, and ultrasonically treat it for 30 minutes. min, let it cool, and then accurately weighed, make up f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com