Polypeptide and application thereof to novel coronavirus detection and antibody or vaccine screening
A coronavirus and virus technology, applied in the field of biomedicine, can solve the problems of long-term human existence, long hidden time, and three-time transmission of the new type of pneumonia virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The synthesis of embodiment 1 polypeptide
[0055] Design the polypeptide sequence according to Table 1, from the C-terminal to the N-terminal of the sequence, the synthesis steps are as follows:
[0056] 1) Weigh 0.3g of resin into a glass reactor, add DCM (dichloromethane) to swell for 30 minutes;
[0057] 2) Take out the DCM, add 0.6g of the first amino acid in the amino acid sequence, add 0.6g of DIEA (diisopropylethylamine), DMF (dimethylformamide), DCM, react by bubbling nitrogen for 60 minutes, and take out The reaction solution was washed three times by adding DMF and MEOH;
[0058] 3) Add 0.6 g of the second amino acid in the amino acid sequence to the reactor, add HBTH (1-hydroxy-benzotrichlorazole tetramethylhexafluorophosphate) and DIEA, react by bubbling nitrogen for 30 minutes, and wash off the liquid. Ninhydrin detection followed by capping with pyridine and acetic anhydride. Finally, wash, add an appropriate amount of decapping solution to remove the ...
Embodiment 2
[0064] The preparation of embodiment 2 polypeptide chips
[0065] Select the three-dimensional D-modified glass slide as the substrate of the polypeptide chip;
[0066] All the polypeptide fragments synthesized in Example 1 and the positive control were spotted on the surface of the slide by a microarray spotter.
Embodiment 3
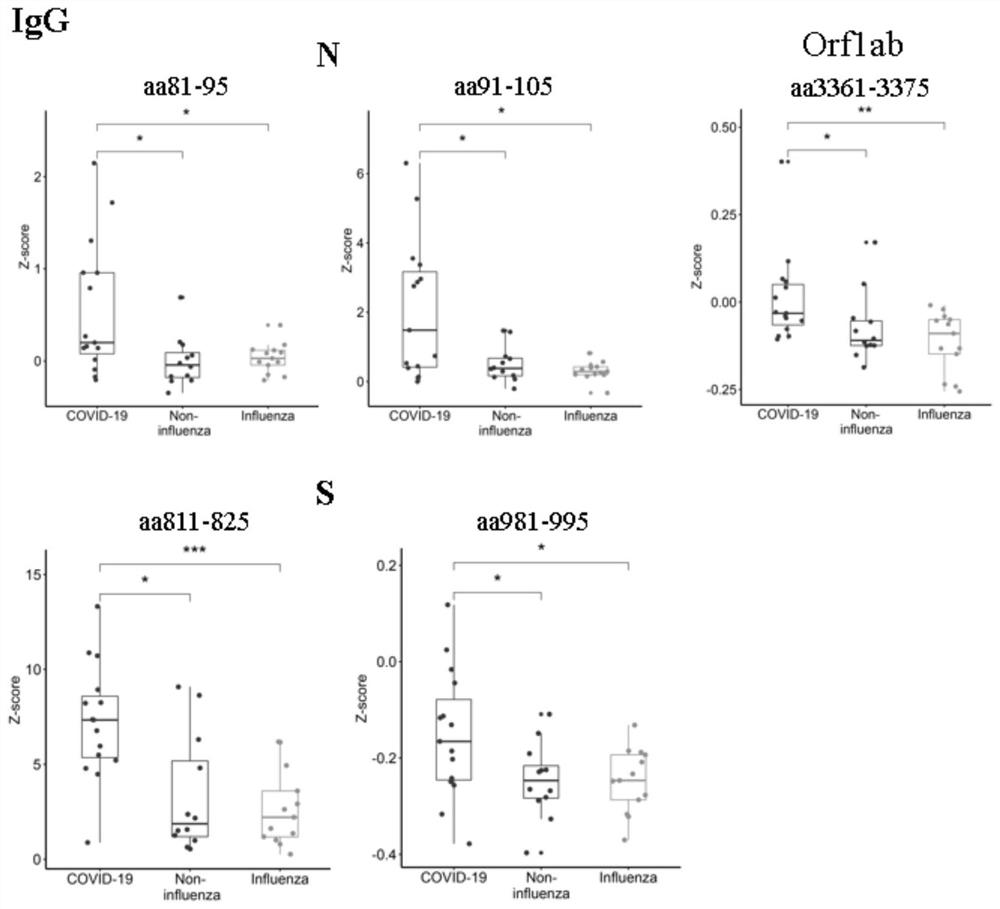
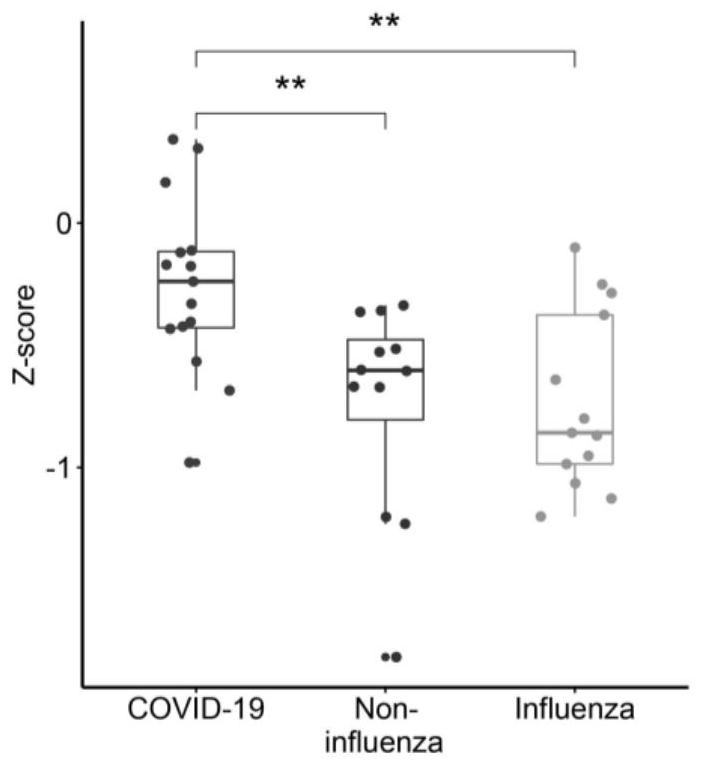
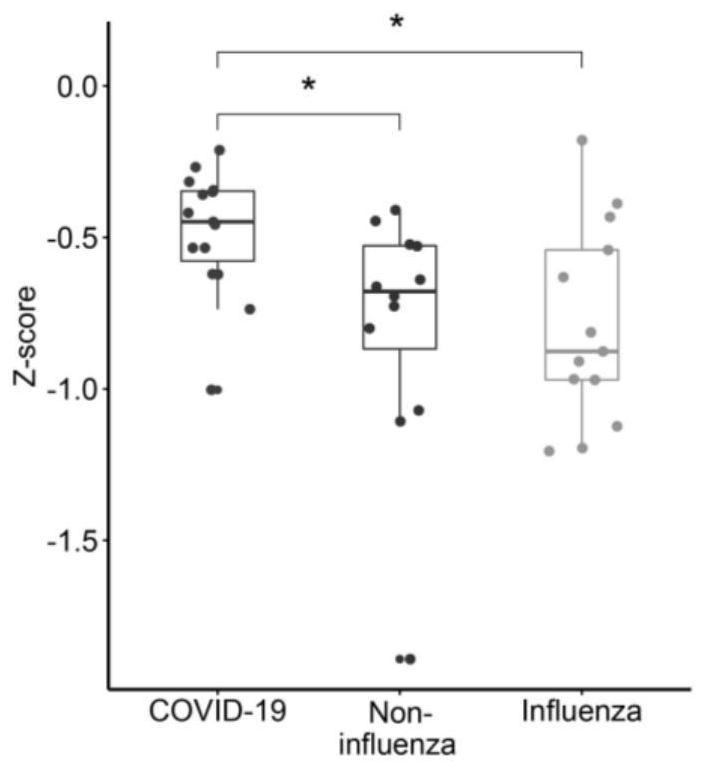
[0067] Example 3 Polypeptide chip detects effectiveness test of SARS-CoV-2 protein antibody
[0068] Sample collection:
[0069] The samples came from Peking Union Medical College Hospital. According to the "Diagnosis and Treatment Plan for Pneumonia Infected by Novel Coronavirus" (trial version 7), patients with mild symptoms of COVID-19 were diagnosed. There were 15 early COVID-19 patients, 13 flu patients with similar symptoms and 12 non-influenza patients.
[0070] Screening of serum samples:
[0071] 1. Sealing: Add a fence to the chip, add 400ul / well 5% milk (0.5g milk powder + 10ml PBST) and seal at room temperature for 0.5h;
[0072] 2. Sample preparation: mix 4ul serum + 400ul 5% milk;
[0073] 3. Adding samples: add the diluted serum sample to the peptide chip prepared in implementation 2, 400ul / well, incubate at room temperature for 1h;
[0074] 4. Washing: wash 3 times with 0.05% PBST, 10 min each time;
[0075] 5. Add fluorescent dyes: add Donkey-anti-hIgG-53...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com