Preparation method and application of self-supporting metal sulfide air electrode
A metal sulfide and air electrode technology, applied to battery electrodes, fuel cell half-cells, secondary battery-type half-cells, circuits, etc., can solve the problem of less metal sulfides, electrode side reactions, and reduced battery life and other issues to achieve low cost, improve performance, and avoid side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
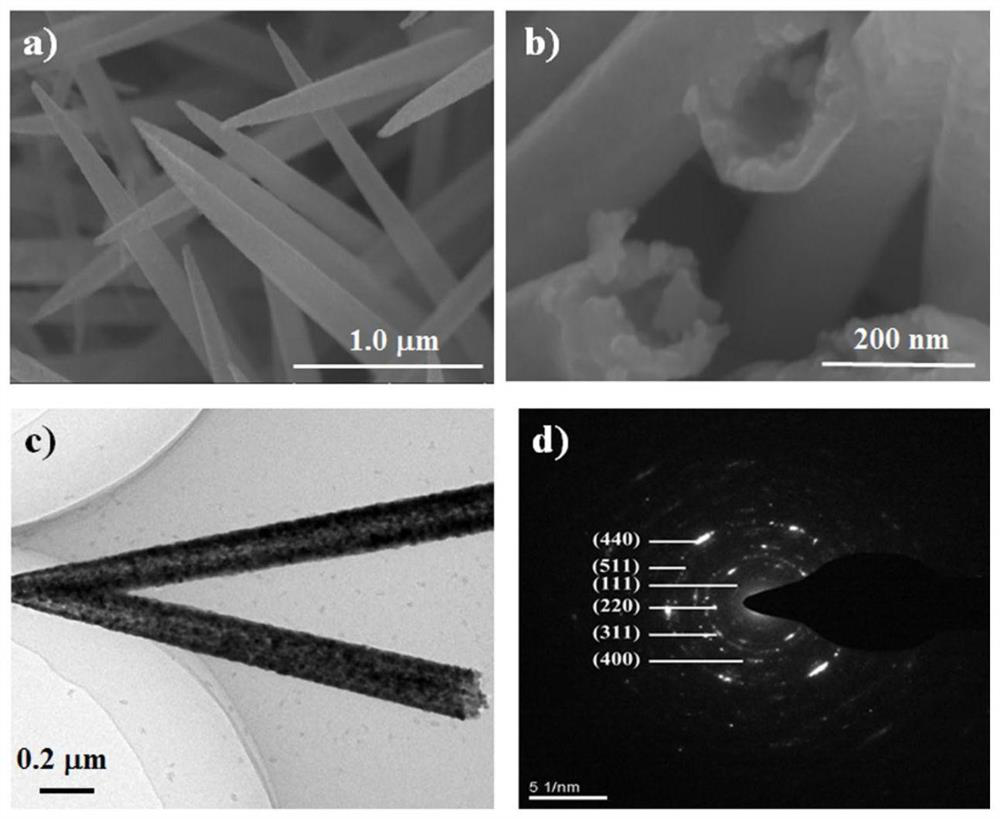
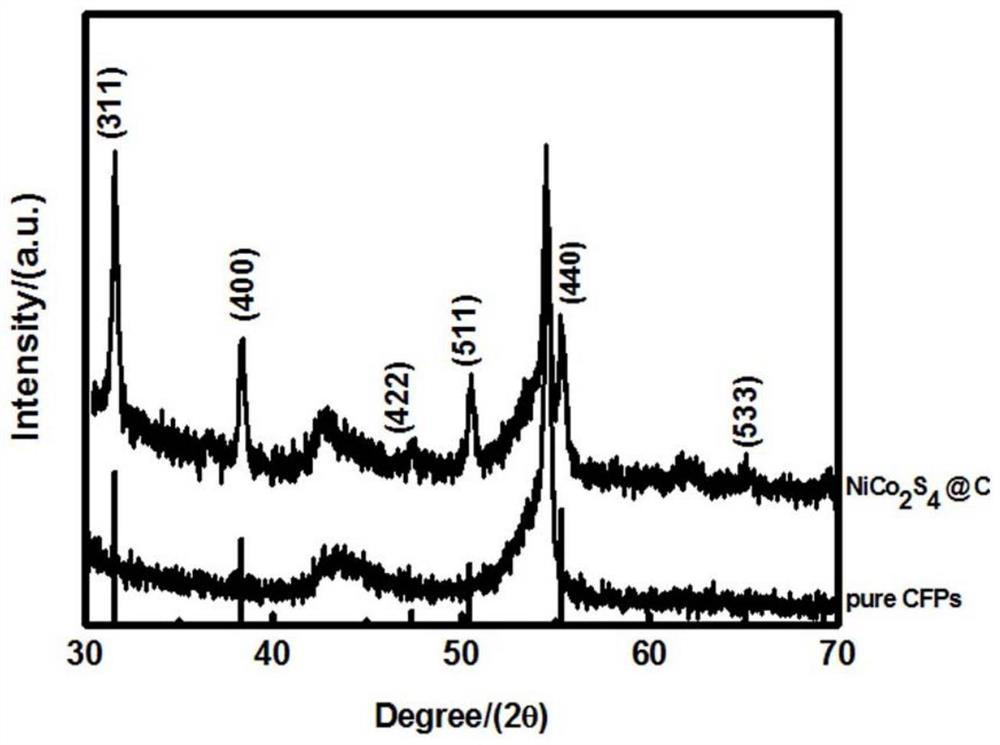
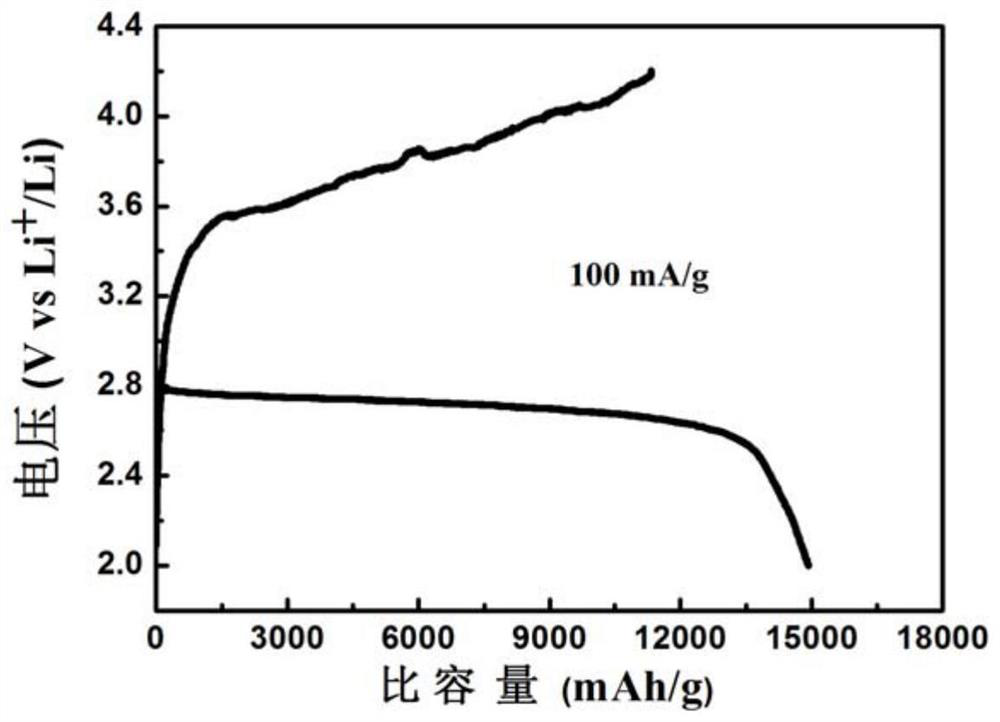
[0024] The preparation method of the self-supporting metal sulfide air electrode of the present invention comprises the following steps: (a) dissolving soluble cobalt salt, soluble nickel salt and precipitant in water to form a mixed solution, and transferring it to a first hydrothermal reaction kettle; The molar ratio of the soluble cobalt salt, the soluble nickel salt and the precipitating agent is 1-3:1:3-5; (b) adding a current collector to the first hydrothermal reaction kettle so that the mixed solution is immersed in the The current collector; then heat the first hydrothermal reaction tank to carry out a hydrothermal reaction to grow a nickel-cobalt precursor on the surface of the current collector; (c) place the current collector grown with a nickel-cobalt precursor In the second hydrothermal reaction kettle filled with sulfide aqueous solution, it is heated to carry out hydrothermal reaction in it; (d) the current collector obtained in step (c) is placed in an inert ga...
Embodiment 1
[0029] The present embodiment provides a kind of preparation method of self-supporting metal sulfide air electrode, and it comprises the following steps:
[0030] (a) 2.5mmol cobalt nitrate hexahydrate (Co(NO 3 ) 2 ·6H 2 O, AR), 1.25mmol nickel chloride hexahydrate (NiCl 2 ·6H 2 O, AR) and 4.5mmol urea (H 2 NCONH 2 , AR) dissolved in 35mL H 2 O, stirred to form a uniform pink solution, and the pink liquid was transferred to the first hydrothermal reaction kettle of 50mL;
[0031] (b) Add carbon paper with a diameter of 14mm (current collector, Toray TGP-H-O6O) into the first hydrothermal kettle, seal it and place it at 130°C for 6 hours of hydrothermal reaction; after the hydrothermal treatment, the surface of the carbon paper grows To produce a pink nickel-cobalt precursor, use deionized water and alcohol to alternately clean the carbon paper for 3 to 5 times, and then dry it in a blast drying oven at 60°C;
[0032] (c) Transfer the dried carbon paper with nickel-cobalt...
Embodiment 2
[0036] The present embodiment provides a kind of preparation method of self-supporting metal sulfide air electrode, and it comprises the following steps:
[0037] (a) 1.25mmol cobalt nitrate hexahydrate (Co(NO 3 ) 2 ·6H 2 O, AR), 1.25mmol nickel chloride hexahydrate (NiCl 2 ·6H 2 O, AR) and 3.75mmol urea (H 2 NCONH 2 , AR) dissolved in 35mL H 2 O, stirred to form a uniform pink solution, and the pink liquid was transferred to the first hydrothermal reaction kettle of 50mL;
[0038] (b) Add carbon paper with a diameter of 14mm (current collector, Toray TGP-H-O6O) into the first hydrothermal kettle, seal it and place it at 100°C for 10 hours of hydrothermal reaction; after the hydrothermal treatment, the surface of the carbon paper grows To produce a pink nickel-cobalt precursor, use deionized water and alcohol to clean the carbon paper for 3 to 5 times, and then dry it in a blast drying oven at 100°C;
[0039] (c) Transfer the dried carbon paper with nickel-cobalt precu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com