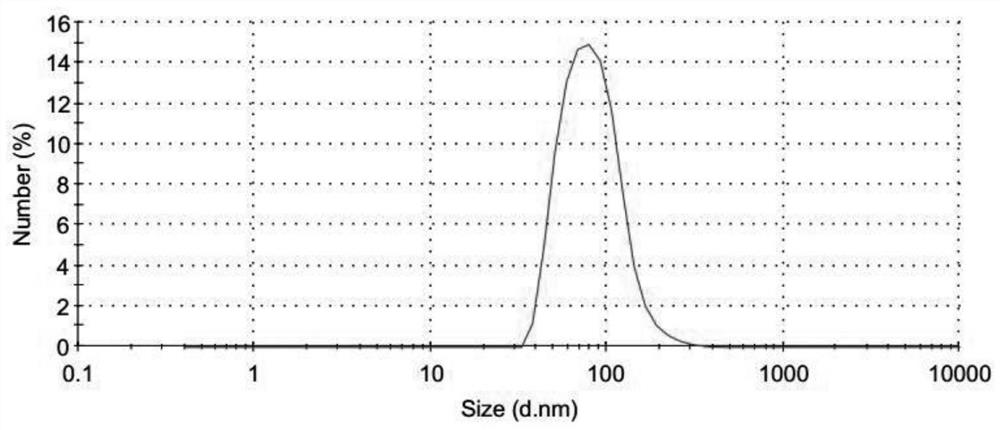
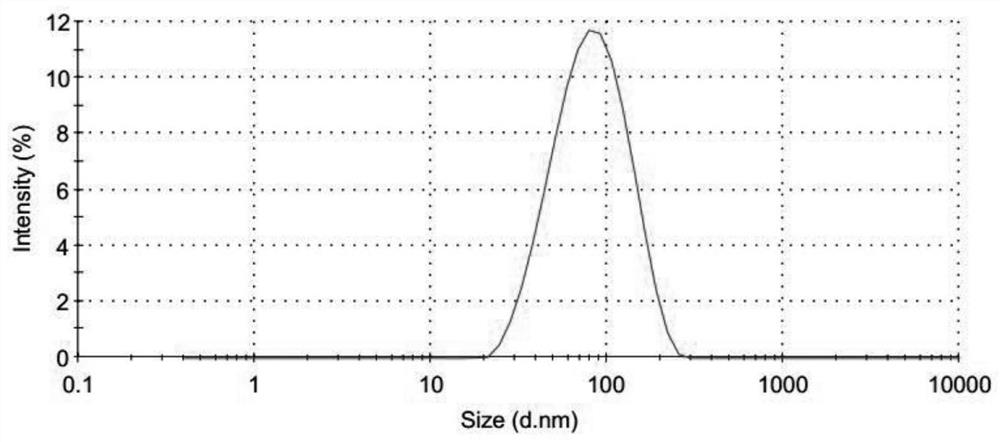
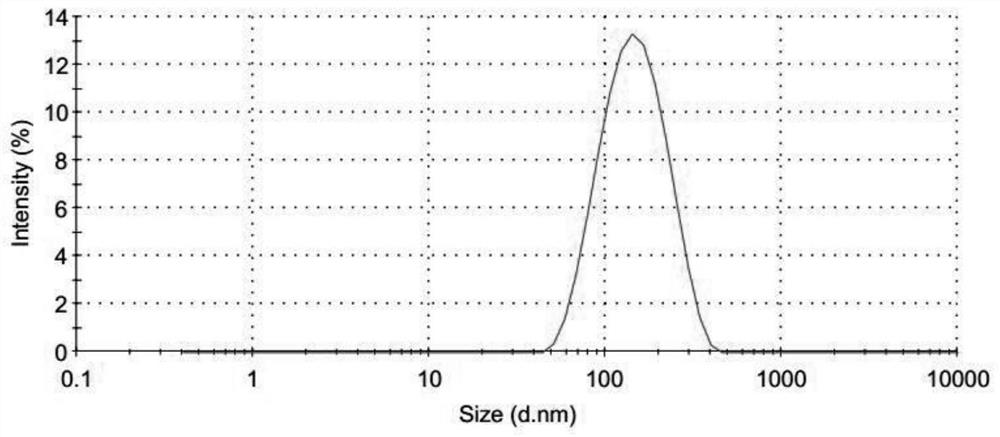
Freeze-dried preparation of vaccine containing aluminum adjuvant, and preparation method and application of freeze-dried preparation
A technology of freeze-dried preparations and aluminum adjuvants, which is applied in the field of vaccine production technology, can solve the problems of high cost, broken cold chain, inconvenient storage and transportation of vaccines, etc., and achieve the effect of low finished product, mild conditions, and simple and rapid preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Preparation of semi-finished products of aluminum hydroxide-chondroitin sulfate-chicken ovalbumin (OVA) nanoparticles: add 60μl of 28mg / ml chondroitin sulfate to 320μl of 100mmol / LPH of 7.7 Hepes buffer, and mix well as phase A; draw 400μl 10mmol / L aluminum sulfate solution, 40μl 1000μg / ml OVA solution, 40μl 100μg / ml oligodeoxynucleotide adjuvant CpG1826 solution mixed uniformly as phase B, add phase B to phase A dropwise under vortexing conditions In the middle, vortex for 30s; add 45mg glucose to the nanoparticle solution to fully dissolve.
Embodiment 2
[0090] Preparation of semi-finished products of aluminum hydroxide-chondroitin sulfate-HBsAg nanoparticles: add 60μl of 30mg / ml chondroitin sulfate to 400μl of 1000mmol / L Hepes buffer with a pH of 7.6, and mix well as phase A; draw 400μl of 10 mmol / L The aluminum sulfate solution and 400μl of 50μg / ml hepatitis B surface antigen solution are mixed uniformly as phase B. Under vortexing condition, add phase B dropwise to phase A, vortex for 30s; add 63mg trehalose to the nanoparticle solution In, fully dissolved.
Embodiment 3
[0092] Preparation of semi-finished products of aluminum hydroxide-chondroitin sulfate-HBsAg nanoparticles: Add 60μl 28mg / ml chondroitin sulfate to 320μl 90mmol / L Hepes buffer with a pH of 7.6, and mix well as phase A; draw 400μl 10mmol / L Mix the aluminum sulfate solution with 350μl 50μg / ml hepatitis B surface antigen solution and use it as phase B. Add phase B to phase A drop by drop under vortexing conditions, vortex for 30s; add 60mg glucose to the nanoparticle solution, fully Dissolve.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com