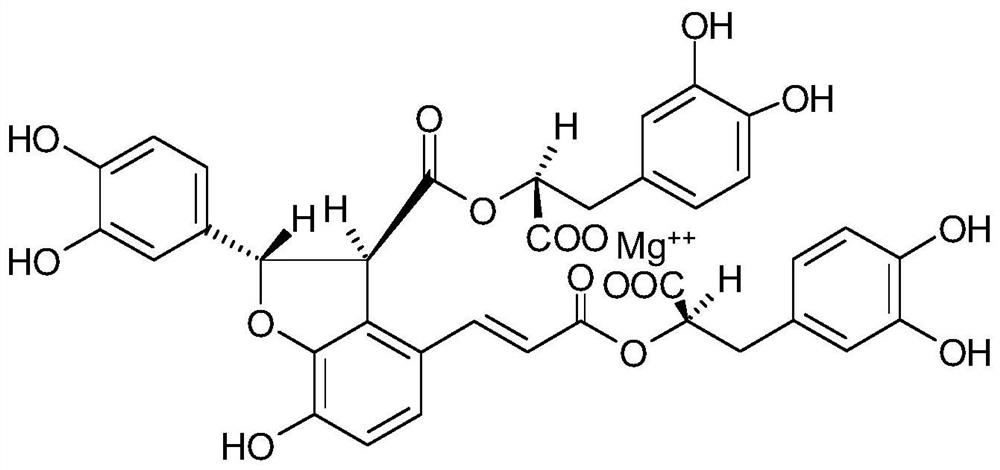
Protective effect and application of magnesium lithospermate B or pharmaceutical composition containing lithospermate B on hepatic ischemia reperfusion
A technology of salvia miltiorrhiza magnesium acetate, liver ischemia, applied in the directions of drug combination, medical preparation containing active ingredients, pharmaceutical formula, etc. Clear protective effect, reduce the effect of complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
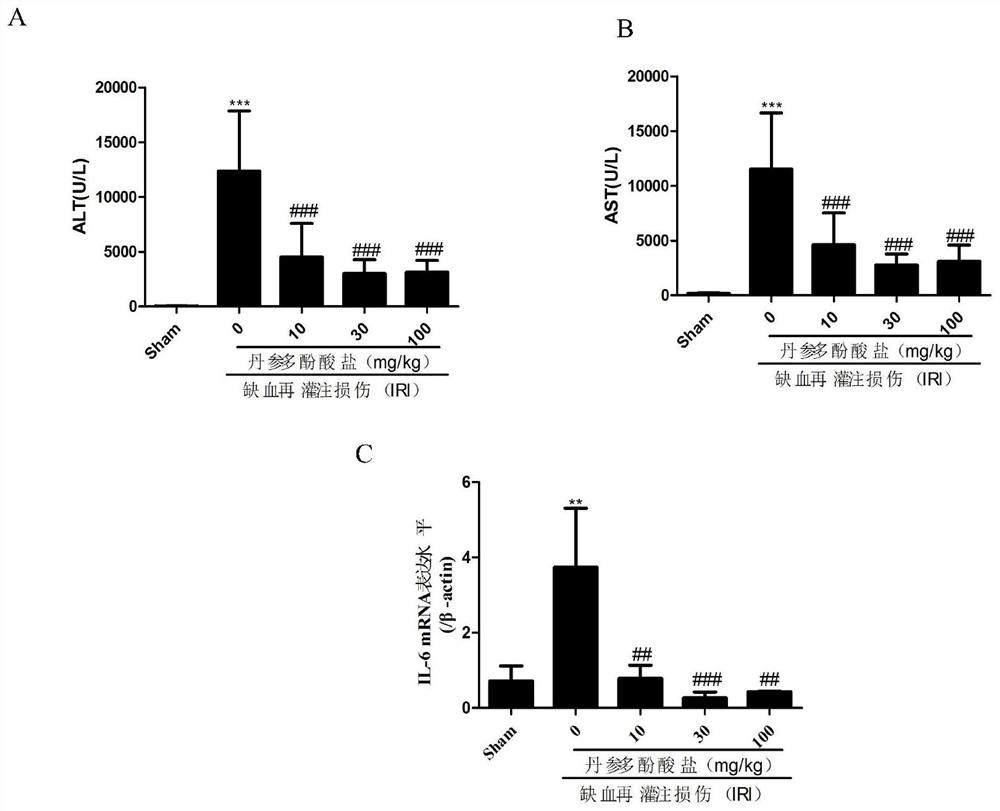
[0025] Example 1 The protective effect of salvianolate on hepatic ischemia-reperfusion
[0026] 1.1 Grouping, administration and experimental methods
[0027] C57BL / 6 mice (purchased from Shanghai Experimental Animal Co., Ltd.) were adaptively fed for three days, and randomly divided into sham operation group (Sham), ischemia-reperfusion injury group (IRI), ischemia-reperfusion injury + salvianolic acid Salvianolate 10mg / kg group, 30mg / kg group, 100mg / kg group, salvianolate (Z20050249) was dissolved in normal saline, ischemia-reperfusion injury + salvianolate 10mg / kg group, 30mg / kg The kg group and the 100mg / kg group were administered three times through the tail vein at 24h, 12h, and 1h before the operation, and each time they were given 10mg / kg, 30mg / kg, and 100mg / kg salvianolate, except for the sham operation group. The rest of the groups were constructed according to literature reports (Ge, M., et al., Brg1-mediated Nrf2 / HO-1 pathway activation alleviates hepatic ischemia...
Embodiment 2
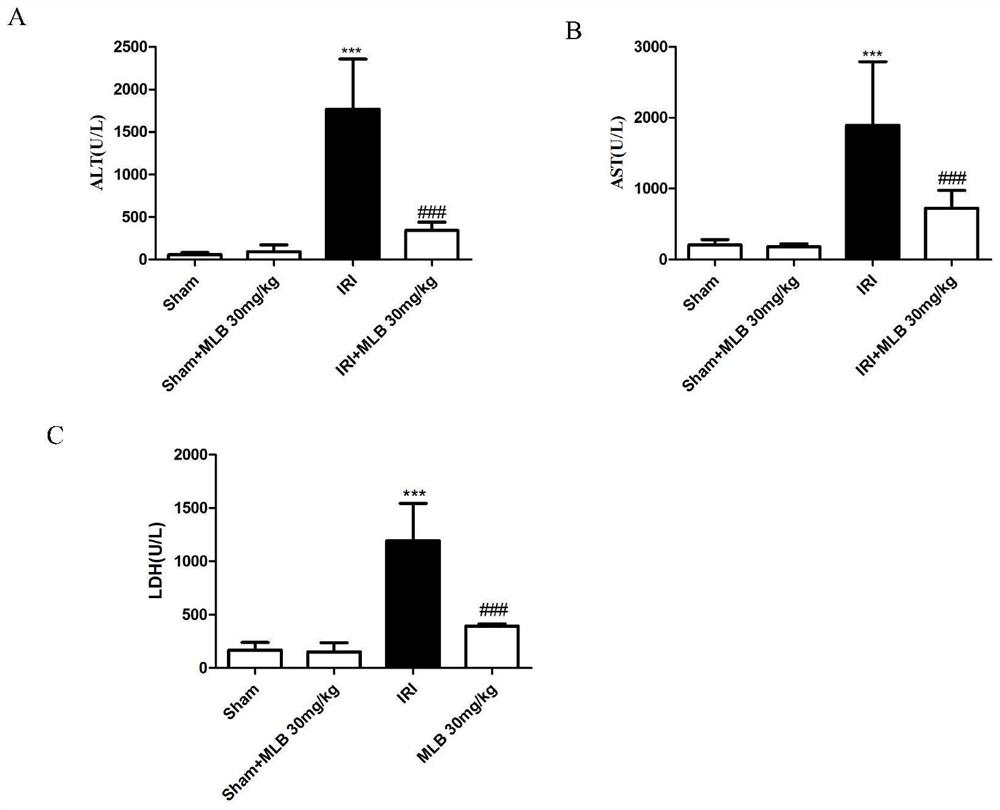
[0033] Example 2 Protective effect and mechanism of magnesium acetate of salvia miltiorrhiza on liver ischemia-reperfusion
[0034] Salvianolic magnesium acetate is the main active ingredient of salvianolic acid salt. Because the above experiments have proved that salvianolate reaches the maximum efficacy dose at a dose above 30mg / kg, the following experiment selects the clinical dose of salvianolic acid magnesium 30mg / kg as the efficacy dose for mechanism research.
[0035] 2.1. C57BL / 6 mice were fed adaptively for three days, and randomly divided into sham operation (Sham) group, ischemia-reperfusion injury (IRI) group, ischemia-reperfusion injury+magnesium salvia acetate (IRI+MLB) 30mg / kg In the group, magnesium acetate of salvia miltiorrhiza (Xuanlijiang Group, Shanghai Institute of Materia Medica, Chinese Academy of Sciences) was dissolved in normal saline, and administered three times via the tail vein at 24h, 12h, and 1h before the operation, 30mg / kg each time. Except ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com