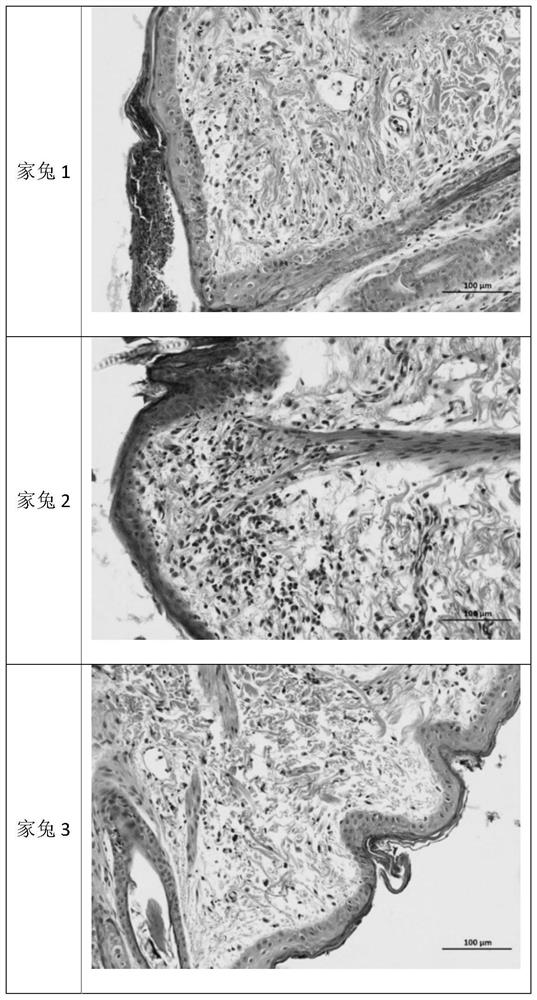
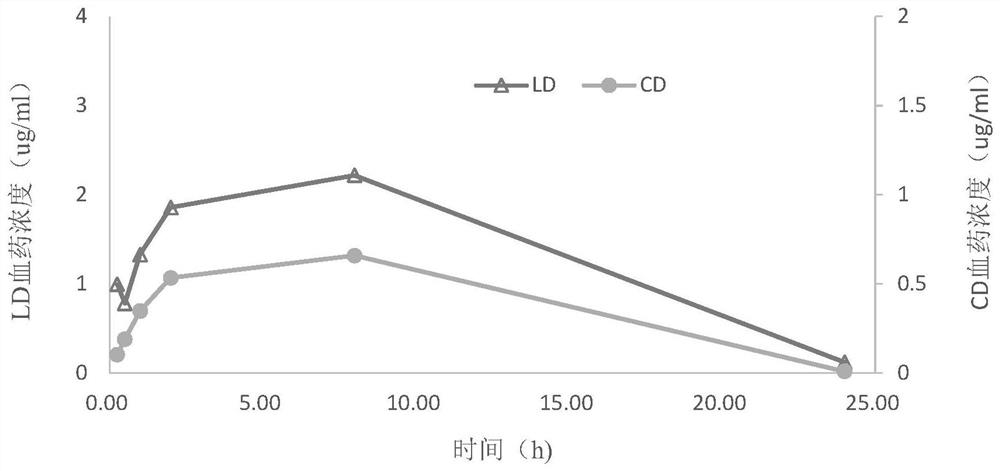
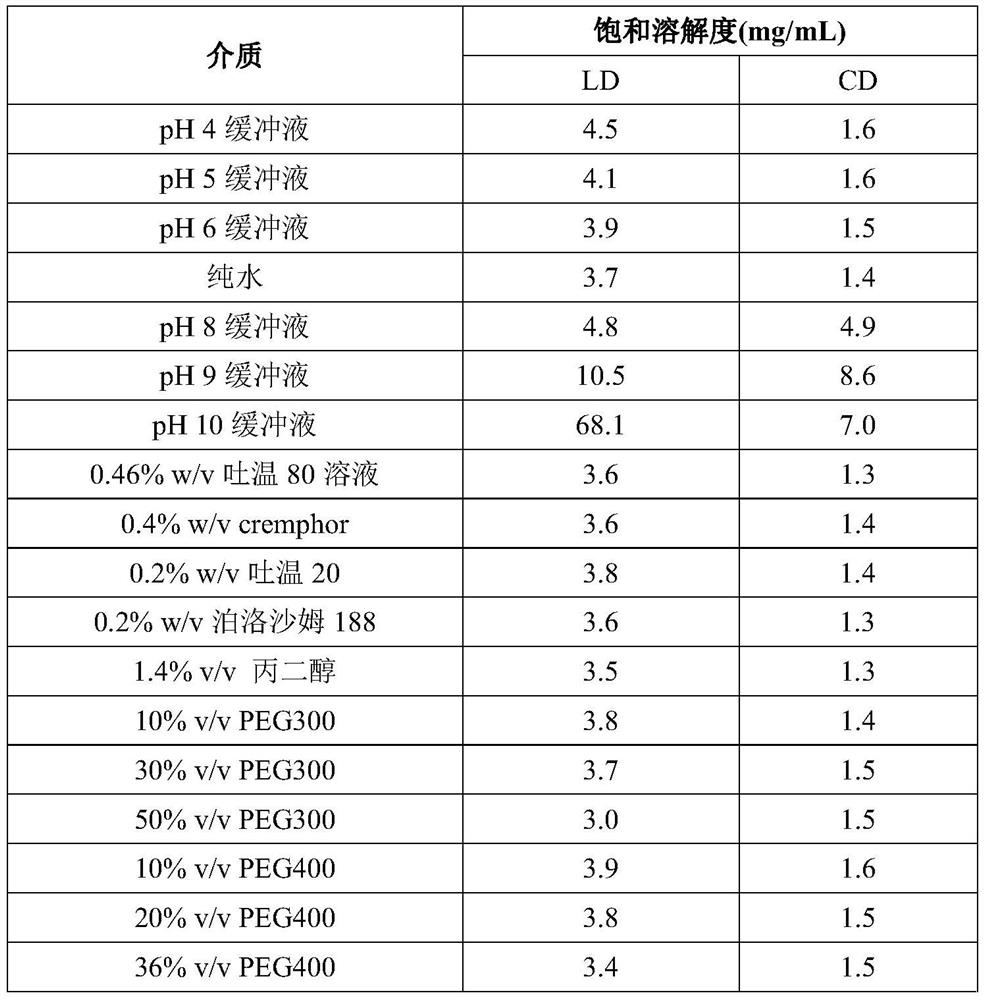
Levodopa preparation and preparation method and application thereof
A technology for levodopa preparations, which is applied in the field of medicine, can solve the problems of inability to prepare high-concentration solution preparations, poor solubility and stability of levodopa, and uneven preparations of levodopa, so as to improve drug compliance and solubility , the effect of volume reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~12 and comparative example 1~6
[0093] Embodiment 1~12 is the levodopa preparation of preferred formula of the present invention;
Embodiment 5
[0138] The levodopa of embodiment 5 (double antioxidant), embodiment 41 (double antioxidant), embodiment 21 (single reduction antioxidant sodium metabisulfite), embodiment 40 (single reduction antioxidant sodium sulfite) After the preparation sample was stored for 3 months (3M), samples were taken to detect the content and related substances. The test results are shown in Table 10.
[0139] Table 10 embodiment 5, embodiment 21 and embodiment 41 long-term stability test result
[0140]
[0141] Investigation result shows, the monoantioxidant sample stability that contains sodium pyrosulfite is better, and the single antioxidant sample LD impurity that contains sodium sulfite has obvious growth; Contrast double antioxidant sample and the double antioxidant sample phase of embodiment 5 Higher impurity than CD, so samples containing sodium metabisulfite monoantioxidant and samples containing sodium metabisulfite and free radical scavenging dual antioxidant are preferred.
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com