HIV-2 recombinant antigen and its preparation method and application
A HIV-2 and recombinant antigen technology, applied in the field of HIV detection, can solve the problems of limited effect and high price, and achieve the effects of increasing the frequency of repeated use, improving specificity, and good freeze-thaw stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Design and Construction of expression vectors Example 1 Recombinant gp36 Antigen
[0077] Using genetic engineering techniques, to be the direction of the dominant epitope region antigen research, the inventors have designed a large number of HIV-2gp36 antigenic fragments, wherein only the gp36-1 (SEQ ID NO.1), gp36-2 (SEQ ID NO .2), gp36-3 (SEQ ID NO.3) and gp36-4 (SEQ ID NO.4) By way of example, the experimental procedure described screening.
[0078] Select 553-668aa region of the full-length HIV-2 antigen, named gp36-1, the method of codon optimized chemical synthesis of nucleotide sequences obtained gp36-1 (SEQIDNO.5); HIV-2 antigen selected Full 538-668aa long region, named gp36-2, the codon-optimized chemical synthesis method to obtain a nucleotide sequence gp36-2 (SEQIDNO.6); 523-668aa selected region of the full-length HIV-2 antigen named gp36-3, the codon-optimized chemical synthesis method to obtain a nucleotide sequence gp36-3 (SEQIDNO.7); and selecting region 5...
Embodiment 2
[0084] Example 2 Rear Grouping GP36 antigen induced expression and purification
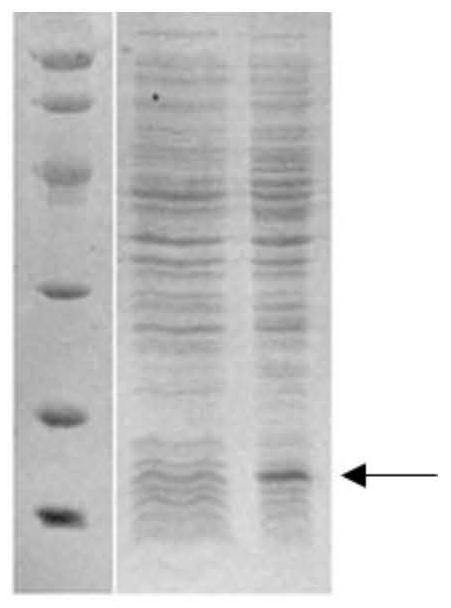
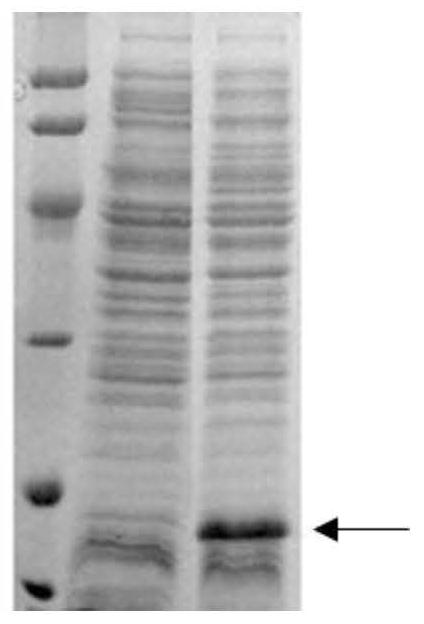
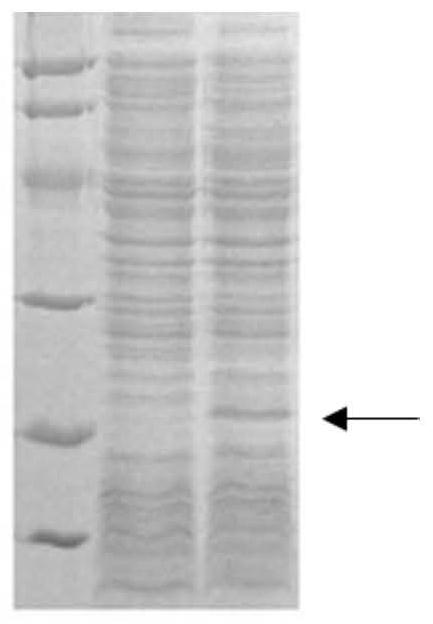
[0085] The positive recombinant plasmid of sequencing, transformed E. coli E.Colibl21 (Rossett) in Escherichia coli was converted to LB agar medium containing 100 μg / ml AMP + and 25 μg / ml CHL +. Under 37 ° C, inverted culture overnight or incubation of 12 to 14 h to long out of single colonies.
[0086] Two single bacteria were collocated to 5 ml of LB liquid medium containing 100 μg / ml amp + and 25 μg / ml CHL +, and 200 rpm was cultured overnight. The next day, 200 μl of bacteria was transferred to 5 mL of fresh lt of LB liquid medium containing 100 μg / ml amp + and 25 μg / mlCHL +, 200 rpm was cultured to OD600 about 0.8. Take 1 ml of bacterial solution as a sample before induction, and IPTG was added to the remaining bacteria to a final concentration of 1 mm, and the oscillation culture was continued at 37 ° C for 4 to 5 h. SDS-PAGE electrophoresis after the end of the induction (see Figur...
Embodiment 3
[0090] Example 3 recombinant GP36 antigen protein replication
[0091] The eluate was collected, loaded into a 3000-3500 DA molecular weight dialysis bag, and complement the destination protein at 2 ~ 8 ° C:
[0092] Buffera: 10mm CB, 0.05% SDS, 10% glycerol, 1mm DTT, 4M urea, pH 10.2;
[0093] Bufferb: 10mm Cb, 0.05% SDS, pH10.2.
[0094] First use buffera (1L) dialysis purposes, from about 2 to 4 h, the equivalent Bufferb (2L) is added to Buffera, and then dialyzes about 2 to 4 h. Thereafter, 1 L of the dialysate containing 2 m urea was poured, and 1 l bufferb was added thereto, and the dialysis was continued for 2 to 4 h. Finally, it is transferred to a new 2L BufferB dialysis, dialysis 3 times, each dialyzing 2 to 4h. After the dialysis is completed, the protein is removed from the dialysis bag.
[0095] The reproached target protein was concentrated with ultrafiltration, then centrifuged at 12000 rpm at 2-8 ° C for 20 min, collected in 15 ml of centrifuge tube, and the antige...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com