Isolator biological purification development verification method and system
A technology of biological purification and verification method, applied in the field of isolator biological purification development verification method and system, can solve the problems of high cost of verification scheme, no SAT confirmation, incorrect confirmation of cycle development parameters, etc., so as to save production cost and manpower Waste of resources, reducing the number of failures and speed regulation, the effect of rigorous program development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0042] The present invention will be further described below in conjunction with the accompanying drawings and specific embodiments.
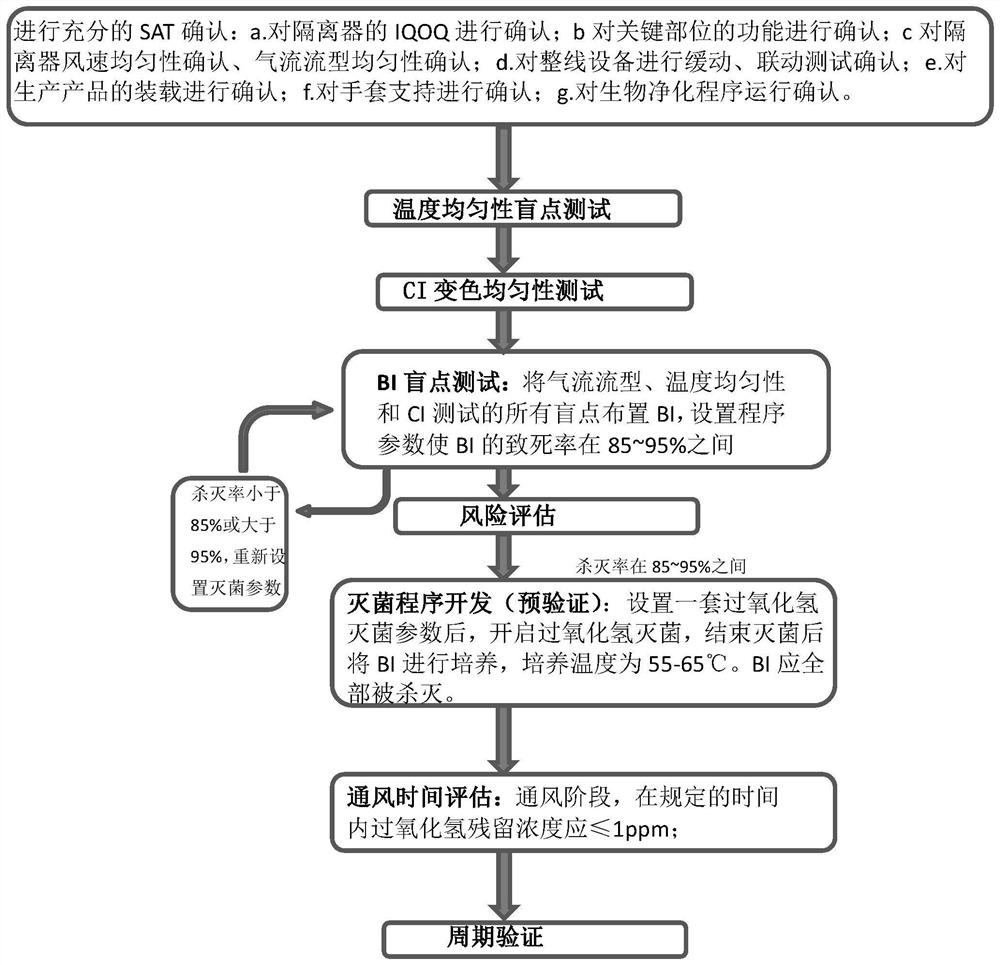
[0043] like figure 1 As shown, the isolator biological purification development verification method of the present embodiment includes steps:
[0044] 1) Perform performance confirmation on the isolator (referred to as SAT confirmation), so that the isolator meets the requirements for the development of biological purification procedures;
[0045] 2) During the development of the biological purification program, through different test items to confirm the poor biological purification effect point in the isolator, and evaluate the final point;
[0046] 3) Pre-verification: After setting a set of hydrogen peroxide sterilization parameters, turn on hydrogen peroxide sterilization, arrange a biological indicator at each final point, and pre-verify the established hydrogen peroxide sterilization parameters. After the sterilization is completed, it i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com