Adeno-associated viral vector for treating III A or III B type mucopolysaccharidosis and application
A virus and recombinant vector technology, applied in the direction of virus/phage, single-stranded DNA virus, introduction of foreign genetic material using vectors, etc., can solve the problems of low efficiency of the central nervous system, little research on gene therapy drugs, and insufficient supply. , to achieve the effect of effective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Embodiment 1 vector construction
[0131] Utilizing the promoter sequence of the present invention, a viral packaging vector is constructed according to conventional methods in the art.
Embodiment 2
[0132] Example 2 Virus Packaging and Genome Titer Detection
[0133] The present invention adopts insect cell SF9 (purchased from ATCC, its number is CRL-1711) as a production cell line, and BestBac baculovirus packaging system to produce recombinant AAV virus vector. For the experimental method used, refer to the operation manual of BestBac Baculovirus Packaging System of ExpressionSystem Company.
[0134] Take an appropriate amount of purified AAV sample, prepare a DNase I digestion reaction mixture as shown in the following table (Table 1), incubate at 37°C for 30 minutes, and incubate at 75°C for 10 minutes to inactivate DNase I.
[0135] Table 1
[0136] AAV samples 5ul 10×DNaseI buffer 5ul DNaseI 1ul RNase-free water 39ul total 50ul
[0137] After the treated AAV purified sample was diluted to an appropriate multiple, the Q-PCR reaction system was configured with reference to the following table (Table 2).
[0138] Table 2
[01...
Embodiment 3
[0150] Example 3 Candidate drug in vitro expression and enzyme activity detection
[0151] 3.1 Detection of reporter gene expression level in vitro
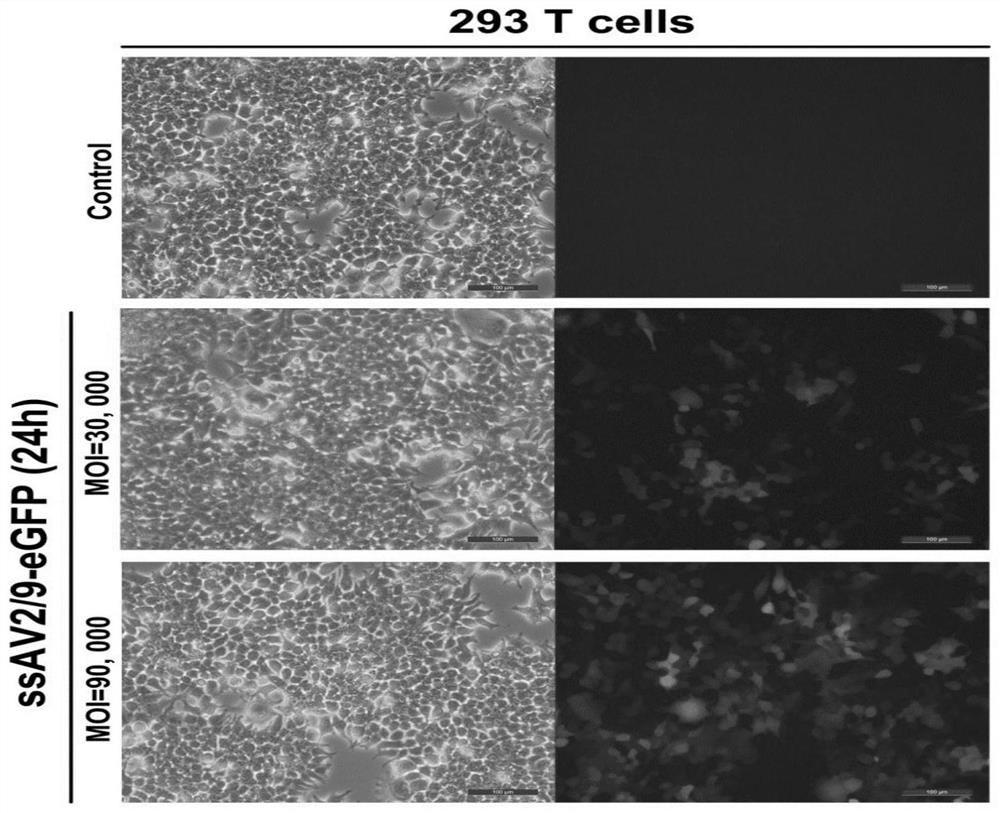
[0152] In order to confirm the effect of ssAAV2 / 9-mediated exogenous gene expression, the packaged ssAAV2 / 9-CAG-EGFP virus was infected with 293T cells at different multiplicity of infection (MOI), respectively, and the infection MOI was 30000 and 90000, respectively, and detected after 24 hours The expression level of green fluorescence, the results are as follows figure 1 As shown, the results showed that ssAAV2 / 9-mediated green fluorescent protein was well expressed in a dose-dependent manner.
[0153] 3.2 In vitro expression level detection of candidate drug target genes
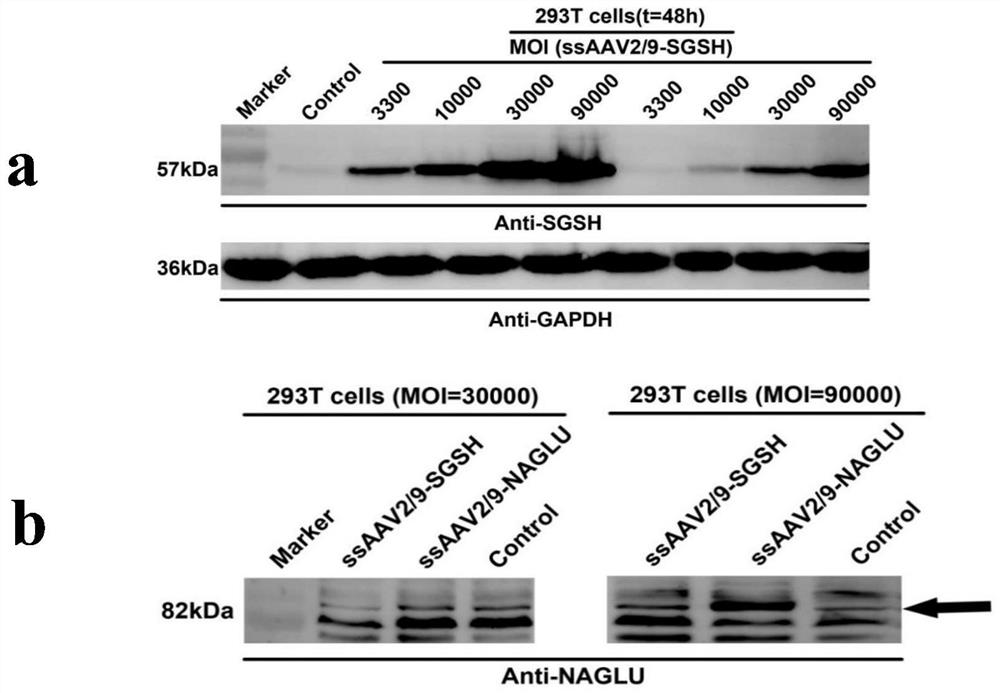
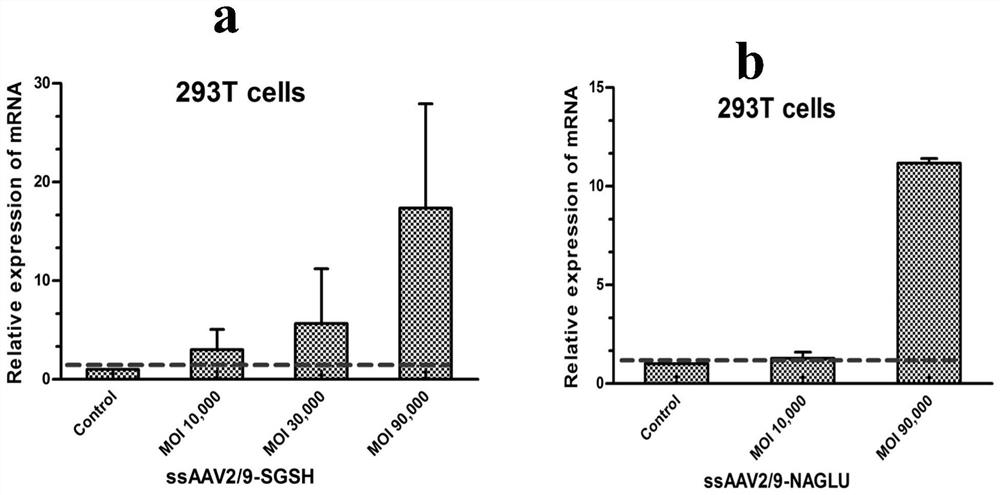
[0154] Next, the expression of the target gene mediated by the ssAAV2 / 9 system was detected, and the constructed and packaged recombinant viruses ssAAV2 / 9-CAG-SGSH and ssAAV2 / 9-CAG-NAGLU were respectively infected with 293T cells at different MOIs, among ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com