Application of lithium complex in hydroboration reaction of nitrile
A complex and ligand technology, applied in catalytic reactions, lithium organic compounds, organic compounds/hydrides/coordination complex catalysts, etc. Simple structure, reduced catalyst dosage, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
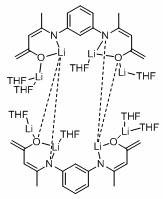
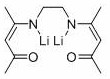
[0026] M-phenyl bridged β-ketimine ligand (L ph H 2 )Synthesis
[0027]
[0028] Add 150 ml of absolute ethanol, 10.8 g of m-phenylenediamine (100 mmol), 20.5 mL of acetylacetone (200 mmol), and catalytic amount of p-toluenesulfonic acid into a three-necked flask. Heat to reflux for 24 hours to obtain a reddish brown liquid and light The yellow solid mixture was filtered with suction, and the solid was recrystallized with absolute ethanol to obtain 24.5 g of light yellow needle crystals, with a yield of 90%, which was ligand L ph H 2 . 1 H NMR(400 MHz, CDCl 3 ): δ 12.47 (2H, s, NH), 7.32-7.27 (1H, m, ArH), 6.94-6.91 (2H, m,ArH), 6.86 (1H, s, ArH), 5.21 (2H, s, CH=C( CH 3 )N), 2.10 (6H, s, CH 3 ), 2.01 (6H,s, CH 3 ). 13 C NMR (101 MHz, CDCl 3 ): δ 196.54 (COCH 3 ), 159.62 (C=CH), 139.63 (Ar-C), 129.71 (Ar-C), 121.45 (Ar-C), 120.43 (Ar-C), 98.20 (=CH), 29.25 (CH 3 ),19.94 (CH 3 ). HRMS (ESI-MS) calcd. for C 16 H 20 N 2 O 2 [M+H] + : 273.1558, found: 273.1633.
[0029] Deprotona...
Embodiment 1
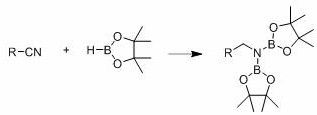
[0035] Example one: [L ph’ Li 4 (THF) 4 ] 2 Catalytic reduction of benzonitrile and pinacol borane
[0036] Under an inert gas atmosphere, add 5.84 mg (0.005 mmol) of catalyst to the reaction flask after dehydration and deoxygenation, and add benzonitrile (64.4 μL, 0.5 mmol) and pinacol borane (159.6 μL) with a pipette. ,1.1 mmol), THF (200 μL), in 60 o After reacting with C for 120 min, use mesitylene (69.6 μL, 0.5 mmol) as the internal standard, stir evenly, pipette a drop into the NMR tube, add CDCl 3 Make a solution. Calculated 1 The H spectrum yield is 96%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) δ 7.31-7.14 (m, 5H, ArCH), 4.23 (s,2H, NCH 2 ), 1.18 (s, 24H, OBpin).
Embodiment 2
[0041] Embodiment two: [L ph’ Li 4 (THF) 4 ] 2 Catalytic reduction of benzonitrile and pinacol borane
[0042] Under an inert atmosphere, add 5.84 mg of catalyst to the reaction flask after dehydration and deoxygenation, and add benzonitrile (64.4 μL, 0.5 mmol) and pinacol borane (159.6 μL, 1.1 mmol) with a pipette. , THF (200 μL), react at room temperature (25℃) for 24 h, use mesitylene (69.6 μL, 0.5 mmol) as the internal standard, stir evenly, pipette a drop into the NMR tube, add CDCl 3 Make a solution. Calculated 1 The H-spectrum yield was 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com