Anti-h5n1 virus cell-entry antibody ptd-3f and its application
A PTD-3F, influenza virus technology, applied in the direction of antiviral agents, antibodies, antibody mimics/scaffolds, etc., can solve the problem of being located on the inside of the virus envelope
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
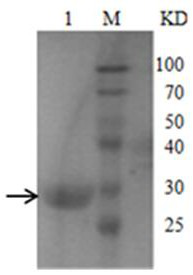
[0027]Example 1 Construction, expression and purification of M1 protein recombinant expression plasmid pET-SUMO-M1 of H5N1 virus M1 protein
[0028] Design and synthesize primers P1 and P2 for M1 protein:
[0029] P1: 5'-atgagtcttctaaccgaggtc-3'; as shown in SEQ ID NO.1 of the sequence listing.
[0030] P2: 5'-CCg gaattc ttaCttgaatcgctgcatctgcact-3'; as shown in SEQ ID NO.2 of the sequence table.
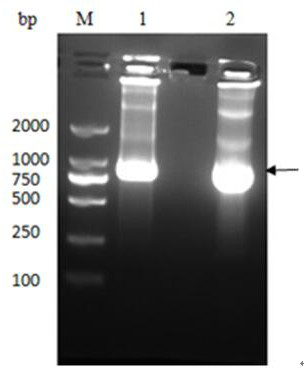
[0031] The M1 protein gene was amplified by PCR using H5N1 cDNA as a template, and cloned into the PET-SUMO vector to construct the plasmid pET-SUMO-M1, which was then transferred into T-shot competent cells and carried out on an agar plate containing kanamycin resistance. initial screening. A single colony was selected and cultured in LB liquid medium; the plasmid was extracted with a plasmid recovery kit and identified by PCR. The product was analyzed by 1% agarose gel electrophoresis, and a band of about 750 bp was obtained, which was consistent with the inserted target gene,...
Embodiment 2
[0034] Example 2 Screening of phage single-chain antibody library
[0035] Inject all the frozen Tomlinson I and J libraries into 200 mL of 2×TY medium (containing 100 µg / mL Amp and 1% glucose), and culture with shaking at 37°C until the OD600 value is about 0.4. Take out 50 mL of bacteria solution from the solution, add 2×10 11 The helper phage KM13 was placed in a water bath at 37°C for 30 minutes, centrifuged at 3000×g for 10 minutes at 4°C, and the pellet was resuspended with 50 mL of 2×TY medium (containing 100 µg / mL Amp, 50 µg / mL Kan and 0.1% glucose). Suspended and cultured overnight at 30°C with shaking. Centrifuge the overnight product at 4°C, 3500×g for 30 min, collect 40 mL of the supernatant, add 10 mL of ice-cold PEG / NaCl solution (final concentration is 20% PEG-6000, 2.5 mol / L NaCl), mix well and store on ice Place for more than 1 h, centrifuge at 4°C, 3500×g for 30 min, discard the PEG / NaCl solution, resuspend the pellet in 2 mL of PBS, centrifuge at 11600×g, ...
Embodiment 3
[0036] Example 3 Screening of anti-M1-scFv
[0037] Coat the purified M1 protein on a 96-well microtiter plate overnight at 4°C. Discard the supernatant the next day, block with 2% Milk-PBS at 37°C for 2 hours, add the prepared secondary phage antibody library, incubate vigorously at room temperature for 60 minutes, discard the liquid after standing for 60 minutes, and use 0.1% Twenn-20 containing Wash with PBS 10 times, after washing, gently pat dry the remaining liquid in each well, add 50 µL of eluent (5 mg / mL trypsin-PBS) to each well, shake vigorously at room temperature for 10 min, elute phage, and collect at 4 °C save;
[0038] Infect E.coli TG1 with the eluted phage, spread on TYE plates (containing 100 μg / mL ampicillin and 1% glucose) and culture overnight at 37°C. Use the helper phage KM13 to amplify the phage library, and recover the phage by PEG / NaCl; repeat the above process 3 times, a total of 4 rounds of screening;
[0039] Phage infection after selection E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com