A kind of fluticasone furoate liposome suspension and preparation method thereof
A technology of fluticasone furoate and fluticasone furoate, which is applied in the directions of liposome delivery, pharmaceutical formulation, liquid delivery, etc., can solve problems such as limitations, and achieve the effects of high encapsulation rate, favorable drug absorption, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of 10ml liposomes
[0032] Total initial volume = 100ml,
[0033] Ethanol content 30%, lipid composition: DPPC / cholesterol (molar ratio is 1: 1),
[0034] Initial lipid = 0.3 mg / ml, initial fluticasone furoate = 0.01 mg / ml,
[0035] Volume of final product = 10ml,
[0036] Step (1) mix and inject:
[0037] Dissolve 19.6mg of DPPC and 10.4mg of cholesterol directly in 30ml of ethanol in a water bath at 50°C, and then add 1mg of fluticasone furoate to completely dissolve it. Slowly inject the above-mentioned ethanol solution into 50ml of physiological saline under stirring state, and after mixing for 20 minutes, dilute to 100ml with physiological saline to obtain an initial volume of 100ml.
[0038] Step (2) diafiltration concentration step:
[0039] Hook the mixing container onto the peristaltic pump and diafilter cartridge. Diafilter cartridges are concave membrane fibers with a molecular weight cut off of 500,000 Daltons. The product was pumped from ...
Embodiment 2
[0041] Preparation of 10ml liposomes
[0042] Total initial volume = 100ml, ethanol content 30%,
[0043] Lipid composition: DPPC / ergosterol (molar ratio is 1:1), initial lipid=0.2mg / ml,
[0044] Initial fluticasone furoate = 0.01 mg / ml, final product volume = 10 ml,
[0045] Step (1) mix and inject:
[0046] Dissolve 19.6mg of DPPC and 10.4mg of ergosterol directly in 30ml of ethanol in a water bath at 50°C, and then add 1mg of fluticasone furoate to completely dissolve it. Slowly inject the above-mentioned ethanol solution into 50ml of physiological saline under stirring state, and after mixing for 20 minutes, dilute to 100ml with physiological saline to obtain an initial volume of 100ml.
[0047] Step (2) diafiltration concentration step:
[0048] Hook the mixing container onto the peristaltic pump and diafilter cartridge. Diafilter cartridges are concave membrane fibers with a molecular weight cut off of 500,000 Daltons. The product was pumped from the reaction vesse...
Embodiment 3
[0050] Fluticasone furoate liposomes with high encapsulation efficiency
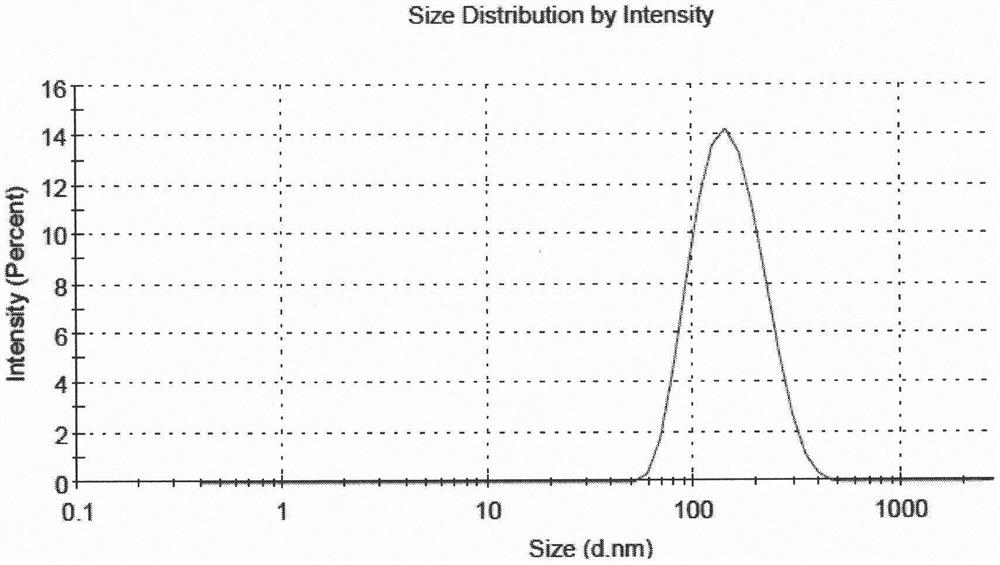
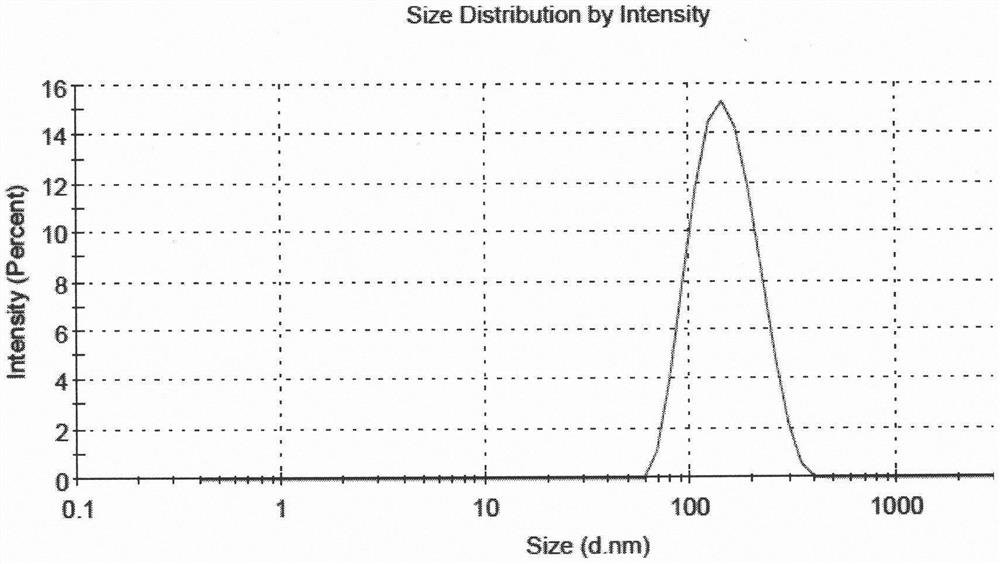
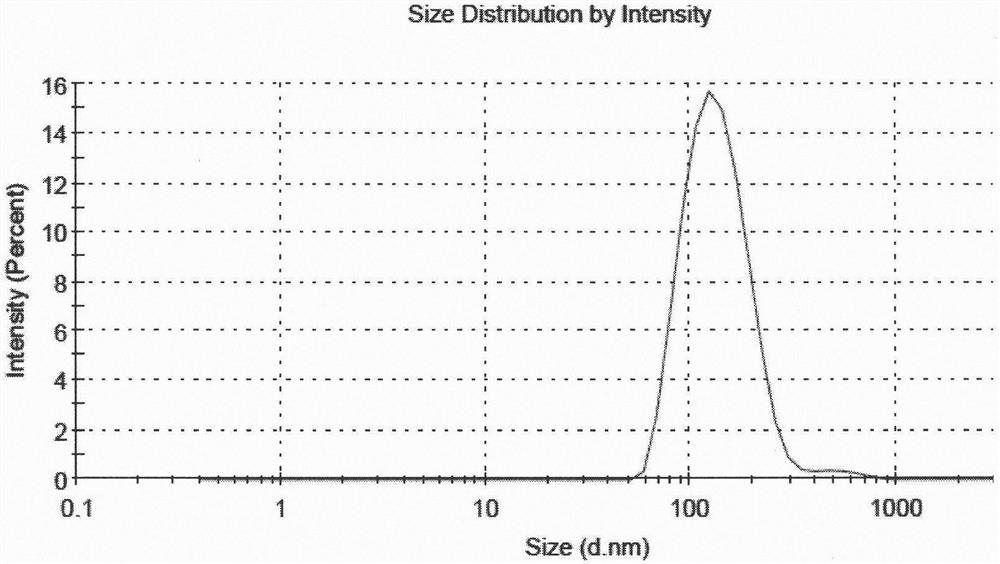
[0051] According to the following steps, 5 parts of liposome suspensions with different drug and lipid ratios and high encapsulation efficiency were prepared, and the encapsulation efficiency of 5 parts of samples was all above 80%, and the encapsulation efficiency of sample 5 The rate is above 90%, and the average particle size is between 130nm and 160nm.
[0052]Sample #1: Weigh 6.5mg of DPPC and 3.5mg of cholesterol in a beaker, add 30ml of ethanol in a water bath at 50°C to dissolve completely, then add 1mg of FF to dissolve completely. While stirring, slowly drop the above solution into 50ml of normal saline with a syringe, mix for 20 minutes, and then dilute to 100ml with normal saline. The product was passed through a hollow fiber membrane at room temperature to remove ethanol and free fluticasone furoate and concentrated to 10 ml.
[0053] Sample #2: Weigh 9.8mg of DPPC and 5.2mg of cholesterol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com