Application of artemisinin compound to promotion of treatment of chimeric antigen receptor T cells and pharmaceutical composition
A chimeric antigen receptor and artemisinin technology, applied in the field of biomedicine, can solve the problems of large side effects, neurotoxicity, and insufficient killing effect of target cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
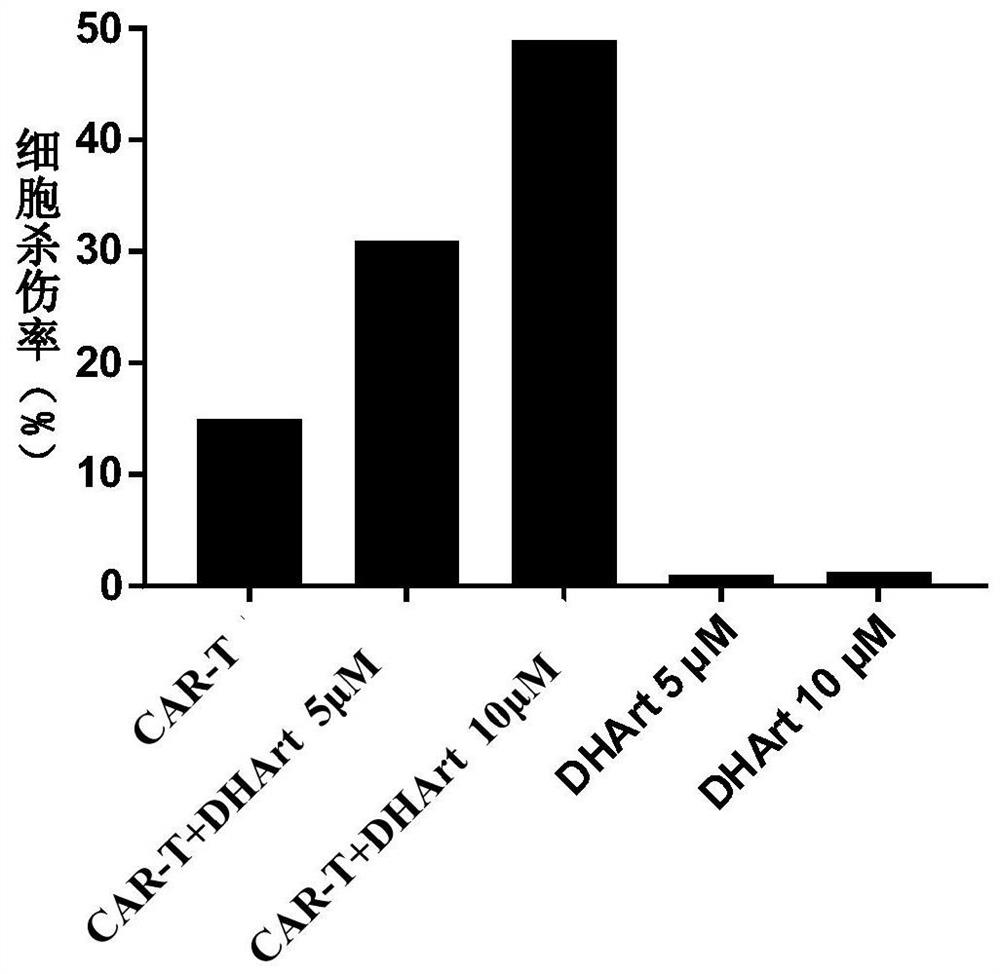
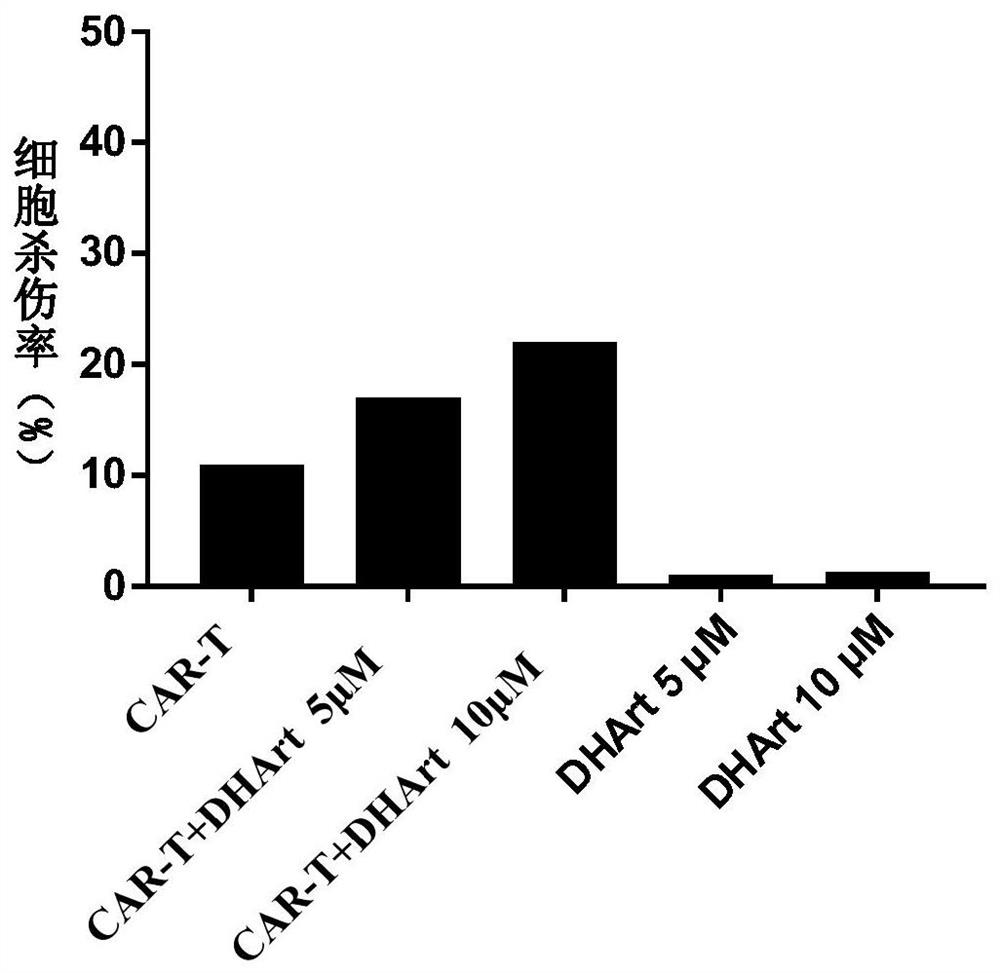
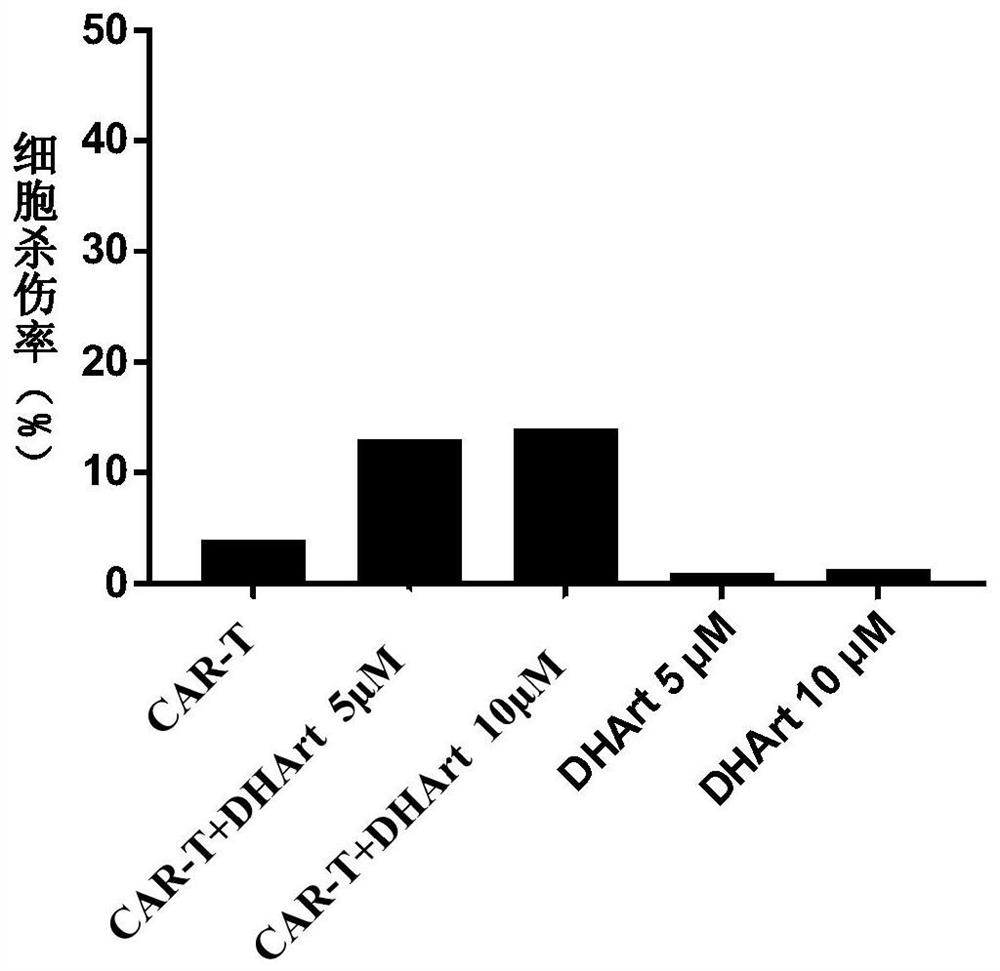
[0034] 1. Resuscitate the CD19-targeting CAR-T cells cryopreserved in liquid nitrogen, and culture the CAR-T cells overnight in complete medium without interleukin IL. Raji cells were cultured overnight in DMEM medium containing 10% FBS and 1% double antibody.
[0035] 2. Collect the overnight cultured CAR-T cells and target cells Raji cells, wash them once with PBS, centrifuge (300g, 4min), and discard the supernatant.
[0036] 3. Resuspend CAR-T cells and Raji cells with KBM 581 basal medium containing no interleukin IL and containing 1% fetal bovine serum, and adjust their cell density. Adjust the cell density of CAR-T to 2×10 6 cells / mL, 1×10 6cells / mL and 5×10 5 cells / mL, the cell density of the target cell Raji is 2×10 5 cells / mL.
[0037] 4. Take a U-shaped 96-well plate, and place 50 μL of CAR-T cells of different densities, 50 μL of Raji cells, and 0.5 μL of DMSO or different concentrations of dihydroartemisinin dissolved in DMSO in each well (the final concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com