Plasma protein marker, detection reagent or reagent tool for diagnosing severe change of new coronal virus pneumonia from mild symptom
A protein, mild technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Mass Spectrometry Analysis of Plasma Samples from Patients with New Coronary Pneumonia
[0035] 1. Experimental materials
[0036] Plasma samples from patients with new coronary pneumonia, from a hospital, including whole blood collected from 10 mild patients (M1-M10, referred to as M) and whole blood collected from 7 severe patients (S1-S7, referred to as S), total 17 whole blood samples. According to the "Diagnosis and Treatment Plan for Pneumonia Infected by Novel Coronavirus (Trial Version 6)" jointly issued by the General Office of the National Health Commission and the Office of the State Administration of Traditional Chinese Medicine, the clinical classification is as follows: mild, mild clinical symptoms, no pneumonia manifestations in imaging ;Severe, meeting any of the following: 1. Shortness of breath, RR≥30 times / min; 2. In a resting state, oxygen saturation ≤93%; 3. Arterial partial pressure of oxygen (Pa02) / inhaled oxygen concentration (Fi02)≤...
Embodiment 2
[0043] Example 2: Machine learning screening for markers from mild disease to severe disease
[0044] 1. Experimental materials
[0045] Mass spectrometry data of 17 plasma samples (see Example 1), Python 3.7 (https: / / www.anaconda.com / ), Scikit learn 0.22.1 (https: / / scikit-learn.org / stable / ). The source code of this experiment is https: / / github.com / Ning-310 / POC-19.
[0046] 2. Experimental process
[0047] (1) To screen for differential proteins, to screen for the absolute value of the fold change (FC) of proteins in the S group and the M group is greater than 0.8, and the two-tailed unpaired Welch T test is less than 0.01 (|log2(FC)|>0.8, unpaired two-sided Welch's t-test, P<0.01).
[0048] (2) Randomly select no more than 5 proteins from the differential proteins to form a potential optimal marker combination (OBC). The initial weight value of each protein is set to 1, and 1000 OBC candidates are set.
[0049] (3) For each candidate OBC, we randomly generate a training d...
Embodiment 3
[0057] Example 3: Validation of OBC Predictor Accuracy
[0058] 1. Experimental materials
[0059] Mass spectrum data of 17 plasma samples (see Example 1), Python 3.7 (https: / / www.anaconda.com / ).
[0060] 2. Experimental process
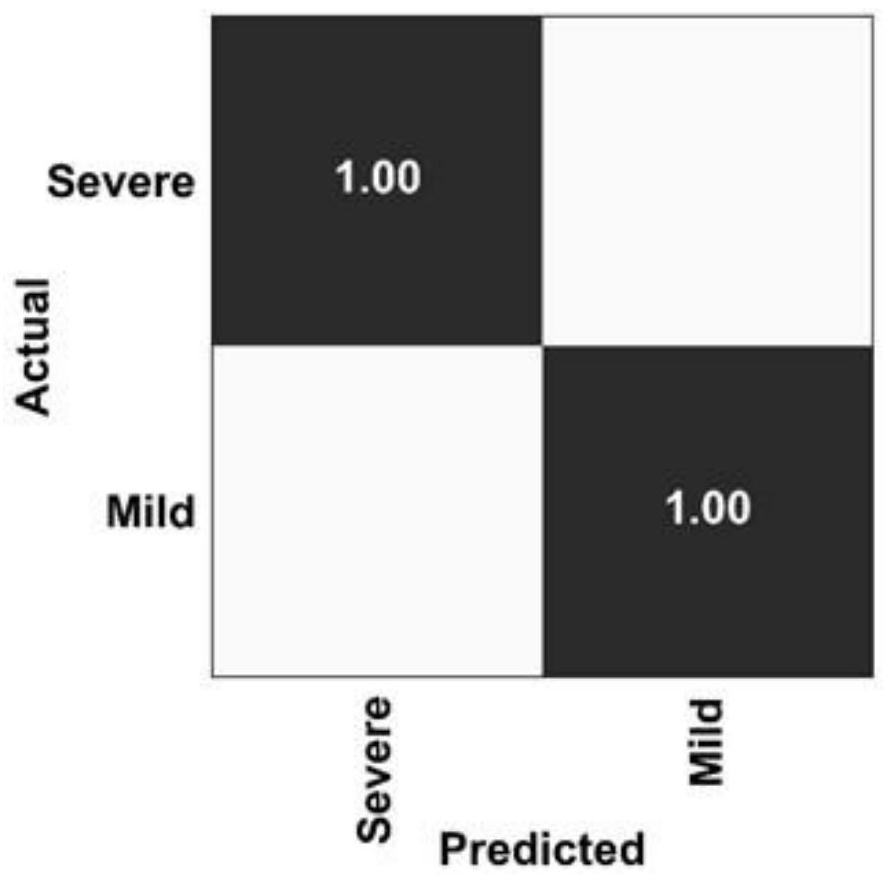
[0061] (1) Identify the true positive (TruePositive, TP), true negative (True Negative, TN), false positive (False Positive, FP) and false negative (False Negative, FN) ratio for chaotic logic analysis.
[0062] (2) Draw the chaotic matrix through Scikit learn 0.22.1 software.
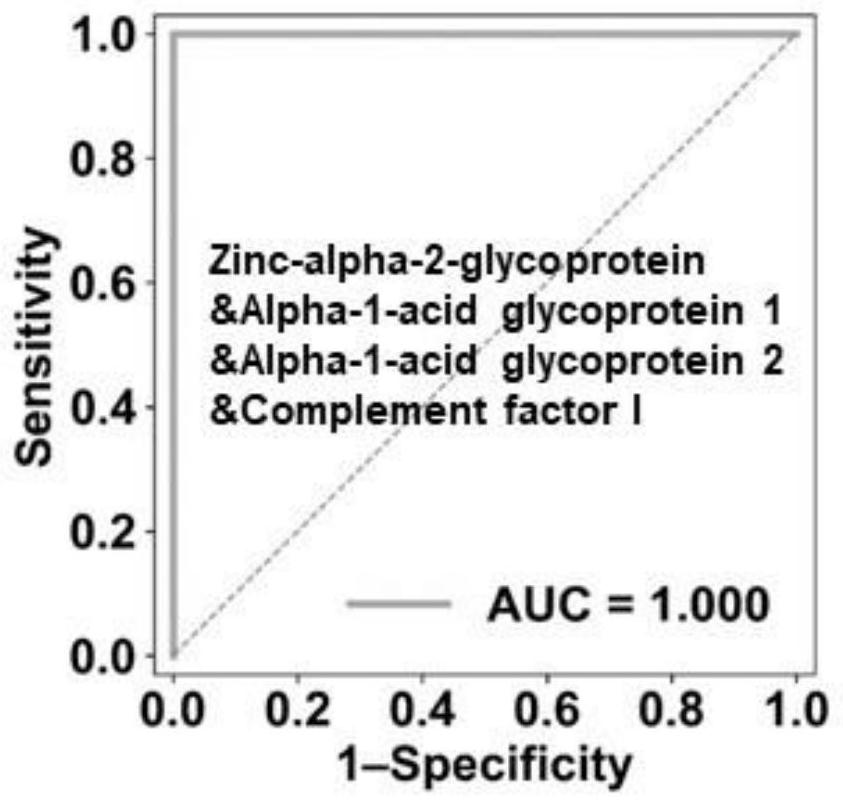
[0063] The result is as image 3 As shown, the OBC combination predicts that the true positive rate of mild to severe disease is 100%, and the false positive rate is 0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com