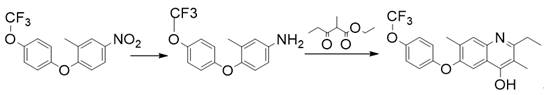
Synthesis method of flometoquin intermediate
A synthesis method and intermediate technology, applied in the field of organic synthesis, can solve problems such as intractable solid waste, and achieve the effects of good environmental protection benefit, low production cost and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add solvent 156.00 g toluene, 3.90 g catalyst Pt / C (3%, humidity 60%), 0.16 g catalyst methanesulfonic acid, 15.60 g (49.80 mmol) 2-methyl-4 -Nitro-1-(4-(trifluoromethoxy)phenoxy)benzene and 8.70 g (54.99 mmol) ethyl 2-methyl-3-oxopentanoate, pressurized to 0.05 MPa with hydrogen, And reflux water separation reaction under this pressure, high performance liquid chromatography monitors the reaction end point, the reaction temperature is finally 123 ℃, after the reaction is completed, the reaction solution is cooled to room temperature, the catalyst Pt / C is recovered by filtration, the filtrate is poured into water, adjusted with hydrochloric acid pH to 5, precipitated white solid, filtered, dried to obtain 16.96 g of product, content 95.5%, yield ≥ 86.2% (with 2-methyl-4-nitro-1-(4-(trifluoromethoxy) phenoxy) benzene).
Embodiment 2
[0019] Add solvent 46.80 g toluene, 0.39 g catalyst Pt / C (3%, humidity 60%), 0.03 g catalyst methanesulfonic acid, 15.60 g (49.80 mmol) 2-methyl-4 -Nitro-1-(4-(trifluoromethoxy)phenoxy)benzene and 8.15 g (51.52 mmol) ethyl 2-methyl-3-oxopentanoate, pressurized to 0.10 MPa with hydrogen, And reflux water separation reaction under this pressure, high performance liquid chromatography monitors the reaction end point, the reaction temperature is finally 135 ℃, after the reaction is completed, the reaction solution is cooled to room temperature, the catalyst Pt / C is recovered by filtration, the filtrate is poured into water, adjusted with hydrochloric acid When the pH reached 5, a white solid was precipitated, filtered, and dried to obtain 17.01 g of the product, with a content of 95.0%, and a yield of ≥ 86.0% (with 2-methyl-4-nitro-1-(4-(trifluoromethoxy) phenoxy) benzene).
Embodiment 3
[0021] Add solvent 78.00 g toluene, 3.90 g catalyst Pt / C (3%, humidity 60%), 0.06 g catalyst methanesulfonic acid, 15.60 g (49.80 mmol) 2-methyl-4 -Nitro-1-(4-(trifluoromethoxy)phenoxy)benzene and 8.38 g (52.97 mmol) ethyl 2-methyl-3-oxopentanoate, pressurized to 0.01 MPa with hydrogen, And reflux water separation reaction under this pressure, the end point of the reaction is monitored by high performance liquid chromatography, the reaction temperature is finally 114 ° C, after the reaction is completed, the reaction solution is cooled to room temperature, the catalyst Pt / C is recovered by filtration, the filtrate is poured into water, adjusted with hydrochloric acid pH to 5, a white solid was precipitated, filtered, and dried to obtain 16.99 g of product, content 95.9%, yield ≥ 86.7% (with 2-methyl-4-nitro-1-(4-(trifluoromethoxy) phenoxy) benzene).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com