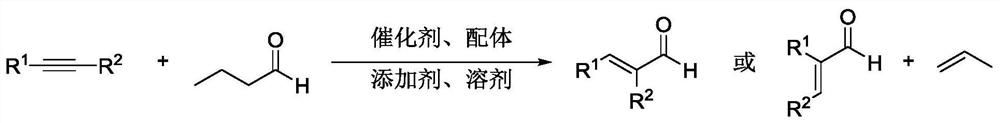
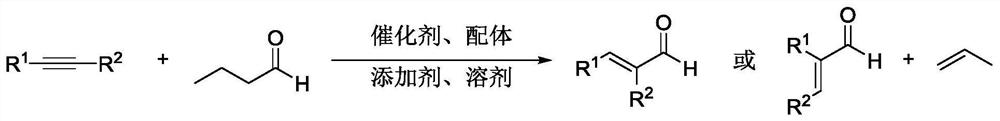
Method for efficiently synthesizing alpha, beta-unsaturated aldehyde without synthesis gas
A synthetic method, unsaturated technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the synthesis of (E)-2-heptyldec-2-enal
[0033] (1) Under nitrogen protection, hexadecane-8-yne (0.4mmol, 88.8mg), n-butyraldehyde (0.2mmol, 18μL), cyclopentadienemethoxyrhodium dimer (4μmol, 1.9mg ), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (8 μmol, 4.6 mg) and 4-nitrobenzoic acid (8 μmol, 1.3 mg) were added to the reaction equipped with a magnetic stir bar In the tube, tetrahydrofuran (100 μL) was added under nitrogen, and reacted at 80°C for 24 hours.
[0034] (2) After the reaction is completed, the reaction tube is cooled to room temperature, dichloromethane is added to dilute the reaction system, then filtered through diatomaceous earth, and washed with dichloromethane, the filtrates are combined, the solvent is removed under reduced pressure, and the residue is purified by silica gel column layer Analysis (petroleum ether / ethyl acetate=200:1, v / v) gave yellow oily liquid (E)-2-heptyldec-2-enal with a mass of 43 mg and a yield of 86%. 1 H NMR...
Embodiment 2
[0035] Embodiment 2: the synthesis of (E)-2-hexyl non-2-enal
[0036] (1) Under nitrogen protection, tetradecane-7-yne (0.4mmol, 77.6mg), n-butyraldehyde (0.2mmol, 18μL), cyclopentadiene methoxyrhodium dimer (4μmol, 1.9mg ), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (8 μmol, 4.6 mg) and 4-nitrobenzoic acid (8 μmol, 1.3 mg) were added to the reaction equipped with a magnetic stir bar In the tube, tetrahydrofuran (100 μL) was added under nitrogen, and reacted at 80°C for 24 hours.
[0037] (2) After the reaction is completed, the reaction tube is cooled to room temperature, dichloromethane is added to dilute the reaction system, then filtered through diatomaceous earth, and washed with dichloromethane, the filtrates are combined, the solvent is removed under reduced pressure, and the residue is purified by silica gel column layer Analysis (petroleum ether / ethyl acetate=200:1, v / v) gave yellow oily liquid (E)-2-hexylnon-2-enal with a mass of 40 mg and a yield of 90%. 1 H NM...
Embodiment 3
[0038] Embodiment 3: the synthesis of (E)-2-butyl hept-2-enal
[0039] (1) Under nitrogen protection, hept-2-yne (0.4mmol, 55.4mg), n-butyraldehyde (0.2mmol, 18μL), cyclopentadienemethoxyrhodium dimer (4μmol, 1.9mg), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (8 μmol, 4.6 mg) and 4-nitrobenzoic acid (8 μmol, 1.3 mg) were added to a reaction tube equipped with a magnetic stir bar , THF (100 μL) was added under nitrogen, and reacted at 80° C. for 24 hours.
[0040] (2) After the reaction is completed, the reaction tube is cooled to room temperature, dichloromethane is added to dilute the reaction system, then filtered through diatomaceous earth, and washed with dichloromethane, the filtrates are combined, the solvent is removed under reduced pressure, and the residue is purified by silica gel column layer Analysis (petroleum ether / ethyl acetate=200:1, v / v) gave yellow oily liquid (E)-2-butylhept-2-enal with a mass of 29 mg and a yield of 86%. 1 H NMR (400MHz, CDCl 3 ):δ=0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com