Carbazole-containing discotic liquid crystal compound and preparation method thereof
A discotic liquid crystal and compound technology, which is applied in the field of carbazole-containing discotic liquid crystal compounds and their preparation, and achieves the effects of enhancing p-p effect, high application value, and simple and fast synthesis route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The compound provided in this example is an intermediate of the compound in general formula (Ⅲ), wherein R 1 for C 12 h 25, Its preparation reaction is as follows.
[0064] R 1 for C 12 h 25 Preparation of intermediate b.
[0065]
[0066]Starting from 3,3',4,4'-tetrakis(dodecyloxy)-1,1'-biphenyl (3.29 g, 3.7 mmol), it was mixed with bromine (0.59 g, 3.7 mmol) to 1:1 molar ratio is weighed in a 250ml round bottom flask, add 150ml of chloroform, put on a constant pressure dropping funnel, add chloroform (20ml) and bromine simple substance into it, put the round bottom flask in room temperature environment In the constant pressure dropping funnel, the solution in the constant pressure dropping funnel is controlled at 2 seconds per drop, and the plate is tracked every 15 minutes. After the reaction is completed, sodium bisulfite is added to terminate the reaction, filtered, spin-dried, and subjected to silica gel column chromatography (elution Agent: V dichlorome...
Embodiment 2
[0069] The compound provided in this example is a compound of general formula (Ⅲ). Wherein R 1 for C 12 h 25, Its preparation reaction is as follows.
[0070]
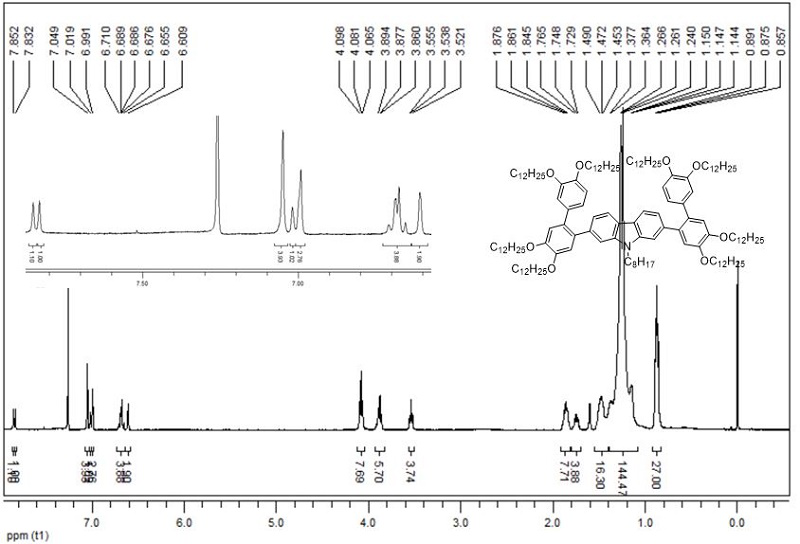
[0071] In a 50 ml reaction tube, b (365 mg, 0.376 mmol): c (100 mg, 0.188 mmol): K 2 CO 3 (779mg, 5.64 mmol): Pd(PPh 3 ) 4 (44mg, 0,0376 mmol)= 2 : 1 : 30 : 0.2 Weigh and add mixed solvent water (3 ml) / THF (10 ml), under the protection of argon, react at 70 ℃ for 48 h, cool to room temperature, extracted with dichloromethane, organic phase with anhydrous MgSO 4 Dry, filter and spin dry. Through silica gel column chromatography (eluent: V dichloromethane: V petroleum ether = 1:3), recrystallization with ethyl acetate and ethanol gave white solid (Ⅲ), (309 mg, yield 80%) .
[0072] H NMR 1 H NMR (CDCl 3 , TMS, 400 MHz), δ:7.85 (s, 1H, ArH), 7.83 (s, 1H,ArH), 7.05 (s, 4H, ArH), 7.02 (s, 1H, ArH),6.99 (s, 1H , ArH), 6.66-6.71 (m,4H, ArH), 6.61 (s, 4H, ArH), 4.07 (t , J = 6.4 Hz , 8H, NCH 2 , OCH 2 ), 3.87...
Embodiment 3
[0074] The compound provided in this embodiment is a compound of general formula (I), wherein R 1 for C 12 h 25, Its preparation reaction is as follows.
[0075]
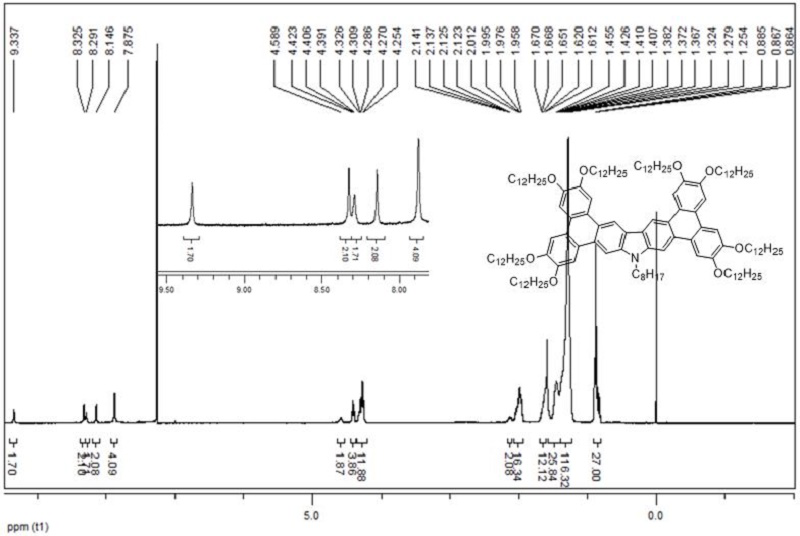
[0076] In a 100ml round bottom flask with compound III (180 mg, 0.087mml): FeCl 3 (85mg, 0.052mml) = 1 : 6 molar ratio, add dichloromethane (60ml), nitromethane (5ml), react at room temperature, track the reaction every 15min, after the reaction is complete, add methanol The reaction was terminated, extracted with dichloromethane, MgSO 4 Dry, filter, spin dry, through silica gel column chromatography (eluent: V toluene: V petroleum ether = 1:1), recrystallize with ethanol and ethyl acetate to obtain yellow-green solid Ⅰ, (90 mg, yield rate 50%).
[0077] H NMR 1 H NMR (CDCl 3 , TMS, 400 MHz), δ: 9.34 (s, 2H, ArH), 8.33 (s,2H, ArH), 8.29 (s, 2H, ArH), 8.15 (s, 2 H, ArH), 7.88 (s, 4H, ArH), 4.59 (s, 2H, NCH 2 ), 4.40 (t, J = 6Hz, 4H, OCH 2 ), 4.25-4.33 (m, 12H, OCH 2 ), 2.12-2.14(m, 2H, CH 2 ), 1.96-2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com