Preparation method of triphenylene-pyrene-triphenylene discotic liquid crystal triplet compound
A discotic liquid crystal, triphenylene technology, applied in the field of discotic liquid crystal triplet compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The compound provided in this example is an intermediate (c) of the compound in general formula (I), and its preparation reaction is as follows:
[0041]
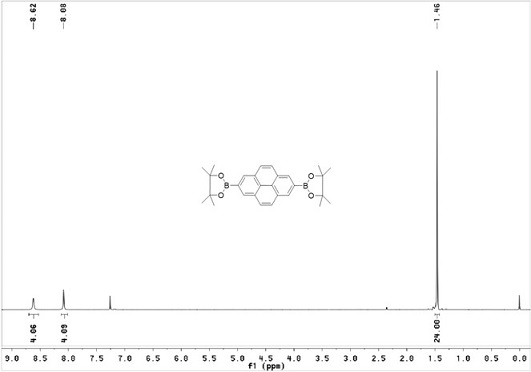
[0042] Using pyrene (1.0 g, 4.944 mmol) as raw material, it was mixed with bis-linked pinacol borate (2.763 g, 10.88 mmol), [Ir(OMe)COD] 2 (0.1707 g, 0.2472 mmol) and dtbpy (0.1354 g, 0.4944 mmol) molar pyrene: double pinacol borate: [Ir(OMe)COD] 2 : dtbpy= 1 : 2.2 : 0.05 : 0.1 was weighed into a 100 ml reaction tube and 30 ml cyclohexane was added, under the protection of nitrogen, reacted at 80°C for 16 h, cooled to room temperature after the reaction, Chloromethane for extraction, organic phase with anhydrous MgSO 4 dried, filtered, spin-dried, and subjected to silica gel column chromatography (eluent: V 二氯甲烷 :V 石油醚 =1:1), recrystallized from ethyl acetate and ethanol to obtain white crystals (c) (1.4 g, yield 62%) .
[0043] H NMR 1 H NMR (CDCl 3 , TMS, 400 MHz), δ:8.62(s, 2H, ArH), 8.08(s, 4H,ArH), 1.46...
Embodiment 2
[0045] The compound provided in this example is R 1 and R 2 Both are -C 6 h 13 Intermediate (b) , Its preparation reaction is as follows:
[0046]
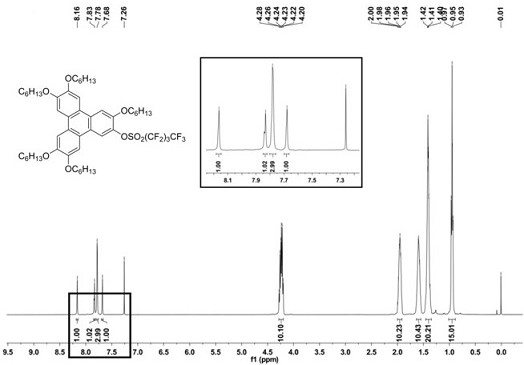
[0047] Starting from 3,6,7,10,11-penta(hexyloxy)-2-hydroxytriphenylene (a) (3.0 g, 4.03 mmol), it was mixed with triethylamine (4.07 g, 40.3 mmol) Weigh it in a 50ml reaction tube at a molar ratio of 1:10, add 20 ml of dichloromethane dried with sintered anhydrous calcium chloride, degas and protect with nitrogen at a temperature of -50°C, and then Then, perfluorobutylsulfonyl fluoride (3.65 g, 12.1 mmol) was added slowly according to the molar ratio of compound (a) and perfluorobutylsulfonyl fluoride being 1:3, and then the reaction was returned to room temperature for 20 h. After the reaction was finished, extract with dichloromethane, and the organic phase was extracted with anhydrous MgSO 4 dried, filtered, spin-dried, and subjected to silica gel column chromatography (eluent: V 二氯甲烷 :V 石油醚 =1:3) separated and purif...
Embodiment 3
[0050] This example provides the preparation of the compound of general formula (I), wherein R 1 and R 2 Both are -C 6 h 13 , the preparation reaction is as follows:
[0051]
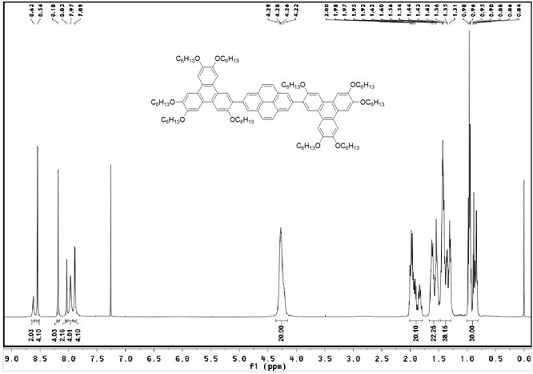
[0052] Weigh (c) (100 mg, 0.2202 mmol), (b) (633.3 mg, 0.6165 mmol), K 2 CO 3 (912.8 mg, 6.605 mmol) and Pd(PPh 3 ) 4 (50.89mg, 0.044 mmol) in a 50 ml reaction tube and add mixed solvent water (3 ml) / THF (10 ml), under the protection of nitrogen, react at 70 ℃ for 48 h, cool to room temperature after the reaction, use Dichloromethane was extracted, and the organic phase was extracted with anhydrous MgSO 4 dried, filtered, spin-dried, and subjected to silica gel column chromatography (eluent: V 二氯甲烷 :V 石油醚 =1:2, recrystallized with ethyl acetate and ethanol to obtain white solid (I) (249.6 mg, yield 68%).
[0053] H NMR 1 H NMR (CDCl 3 , TMS, 400 MHz), δ:8.62(s, 2H, ArH), 8.54(s, 4H,ArH), 8.18(s, 4H, ArH), 8.03(s, 2H, ArH), 7.97(s, 4H , ArH), 7.89(s, 4H, ArH),4.22-4.29(m, 20H, OCH 2 ), 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com