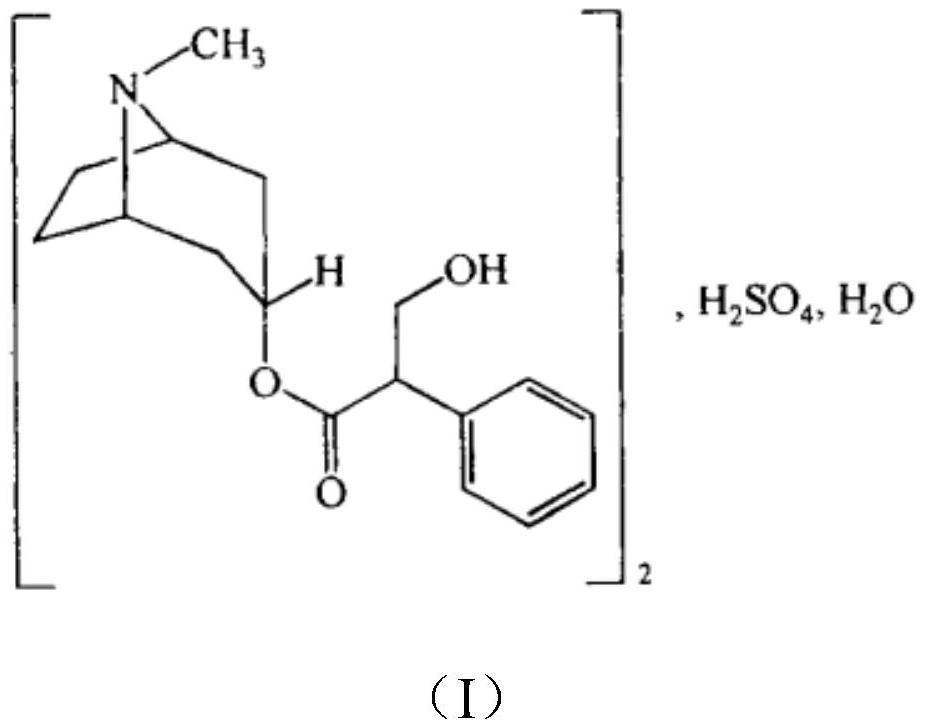
Atropine sulfate eye drops
A technology of atropine sulfate and eye drops, which is applied in the direction of making medicines into special physical or ingestible devices, inorganic non-effective ingredients, muscular system diseases, etc., can solve problems such as unfavorable long-term use and human eye irritation, and achieve It is beneficial to long-term use, reduces the efficacy of the drug, and eliminates the effect of adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
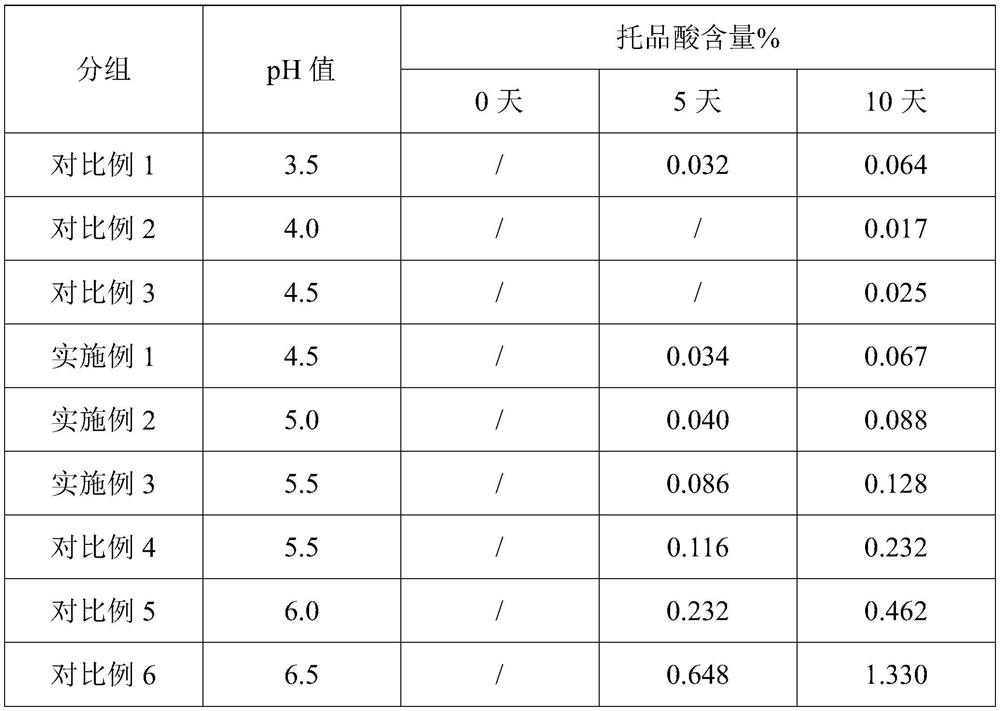
[0028] A kind of atropine sulfate eye drop, its raw material comprises: atropine sulfate 10mg, sodium chloride 0.810g, disodium hydrogen phosphate 0.014g, sodium dihydrogen phosphate 0.750g and water for injection 100ml;
[0029] The preparation process is as follows: in a sterile environment, weigh each raw material, add disodium hydrogen phosphate, sodium dihydrogen phosphate, atropine sulfate, and sodium chloride into an appropriate amount of water and stir to dissolve, then use the remaining water to constant volume and mix to obtain the pH =4.5, the osmotic pressure molar concentration ratio is 0.9, the solution of atropine sulfate concentration is 0.10mg / ml, filters again three times, adopts BFS full-automatic blow-fill-seal all-in-one machine to fill in the eye drop agent bottle, lamp inspection, packing obtains Atropine sulfate eye drops, wherein, the filling volume of atropine sulfate eye drops is 0.5ml / support; the filter membrane pore diameter of the first filtration...
Embodiment 2
[0031] A kind of atropine sulfate eye drops, its raw material comprises: atropine sulfate 10mg, sodium chloride 0.900g, disodium hydrogen phosphate 0.014g, sodium dihydrogen phosphate 0.700g and water for injection 100ml;
[0032] Wherein, the pH of atropine sulfate eye drops=5.0, the osmotic pressure molar concentration ratio is 1.0, and its preparation method is the same as embodiment 1.
Embodiment 3
[0034] A kind of atropine sulfate eye drop, its raw material comprises: atropine sulfate 10mg, sodium chloride 0.990g, disodium hydrogen phosphate 0.014g, sodium dihydrogen phosphate 0.650g and water for injection 100ml;
[0035] Wherein, the pH of atropine sulfate eye drops=5.5, the osmotic pressure molar concentration ratio is 1.1, and its preparation method is the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com