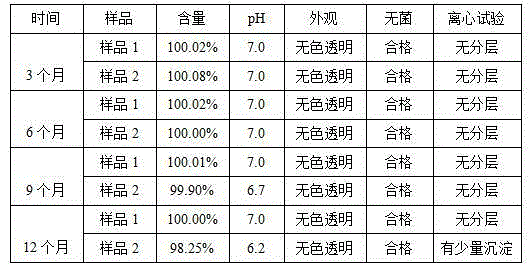
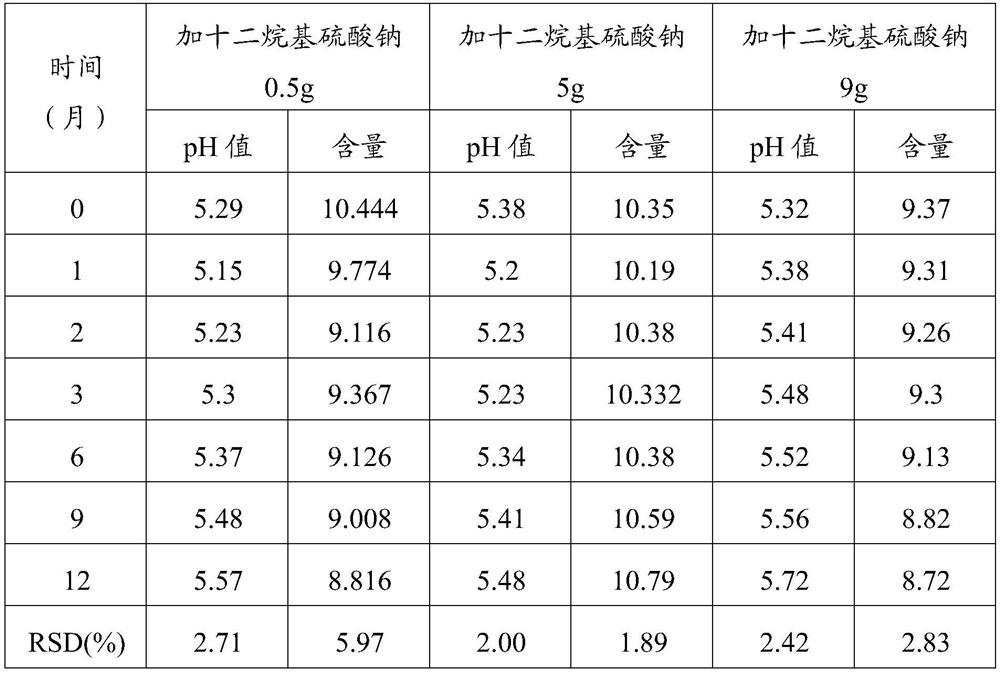
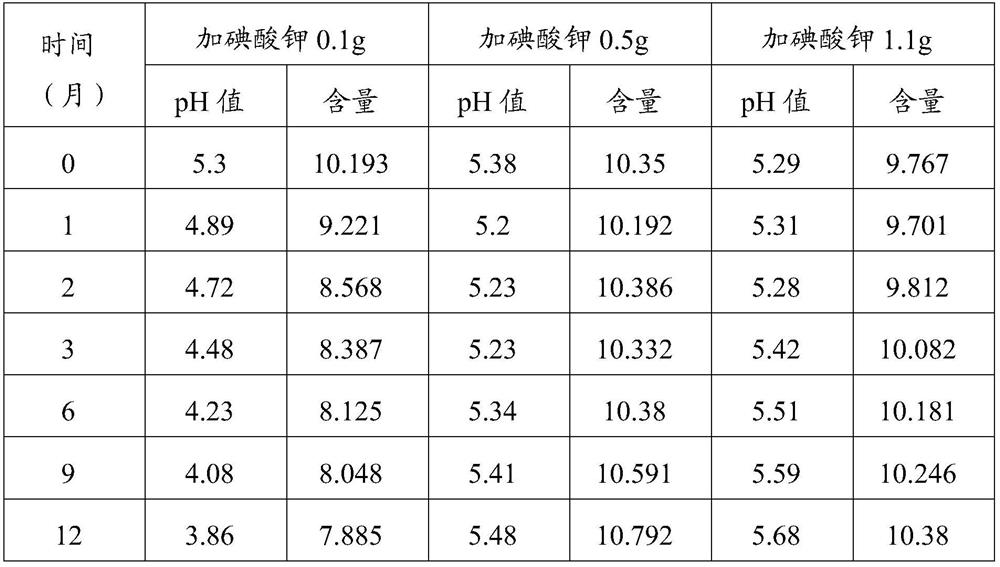
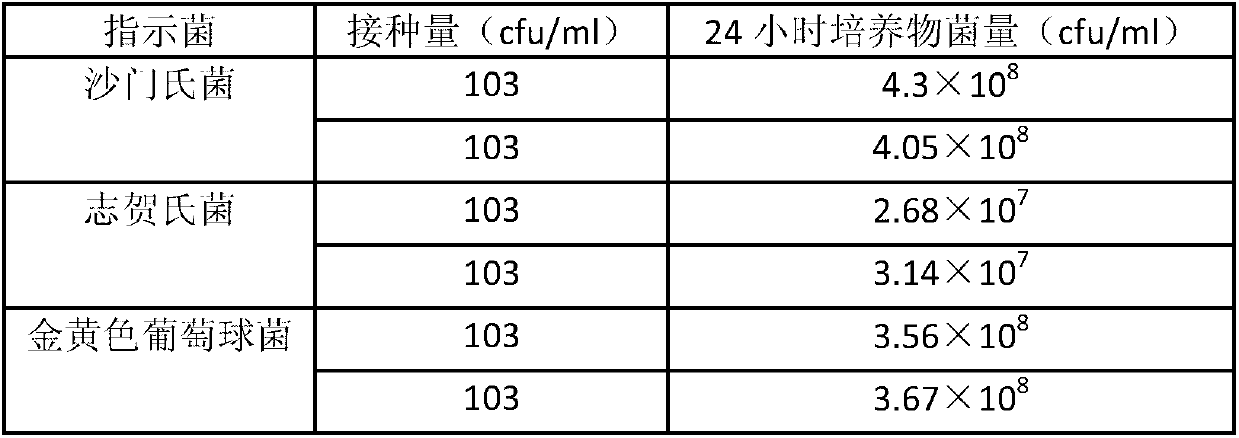
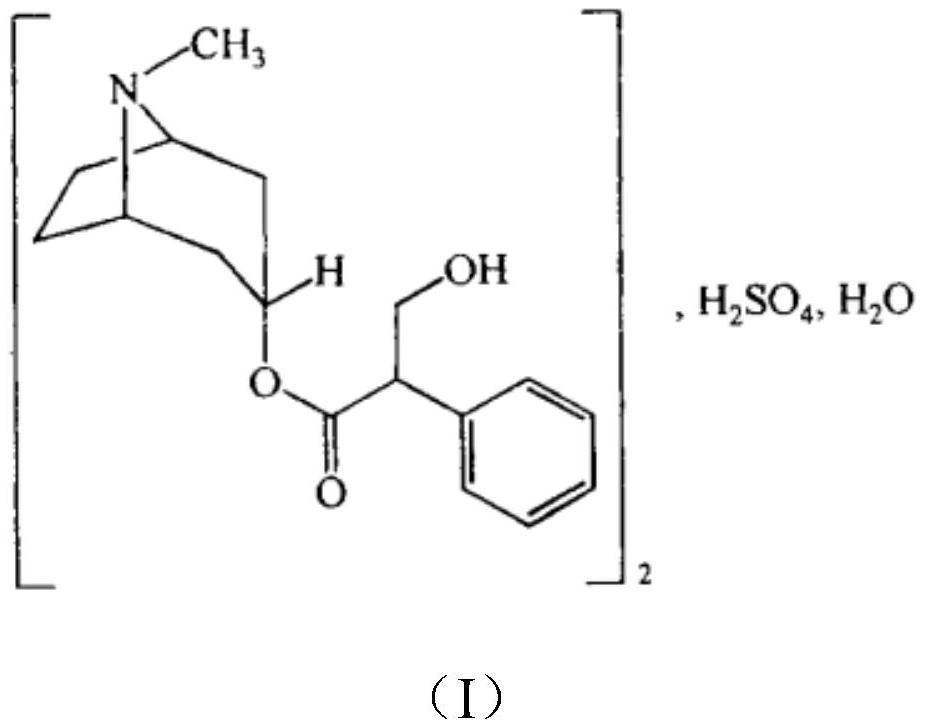
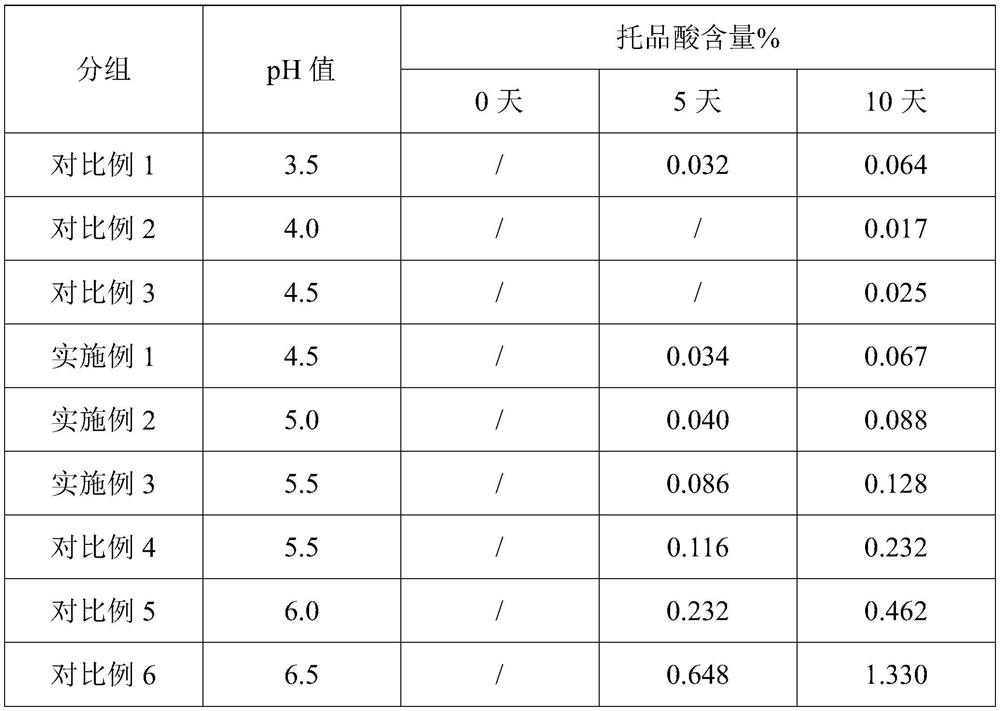
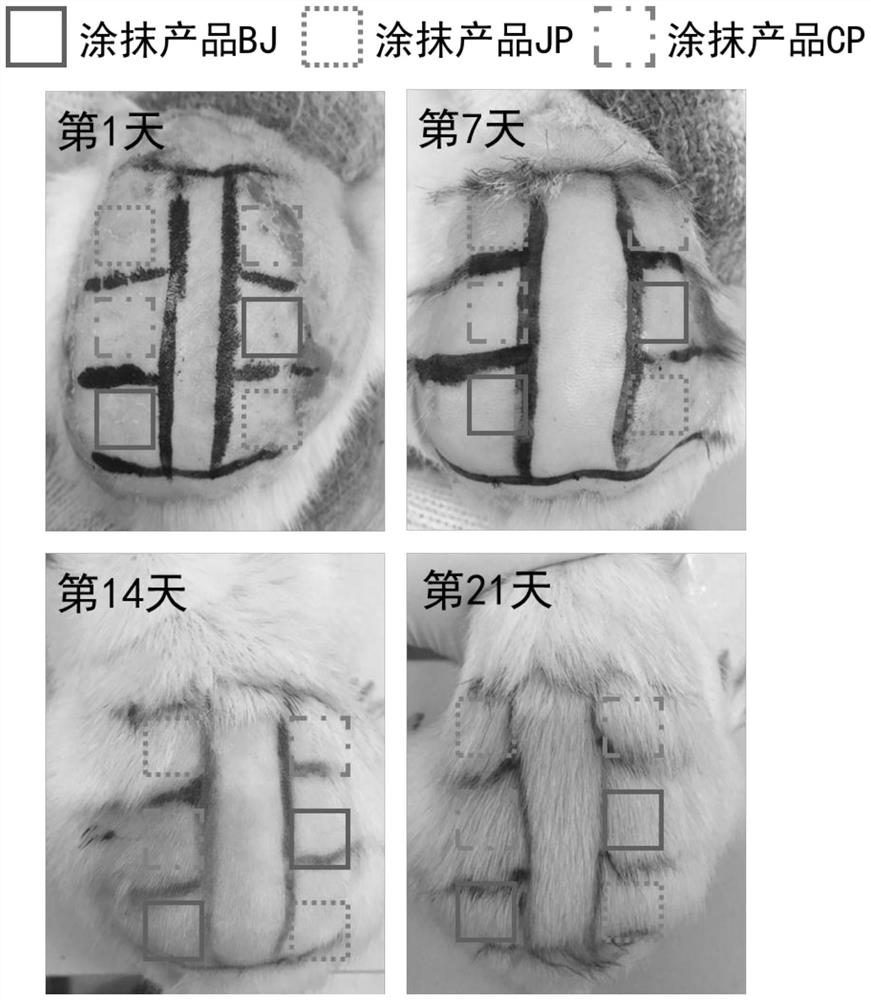
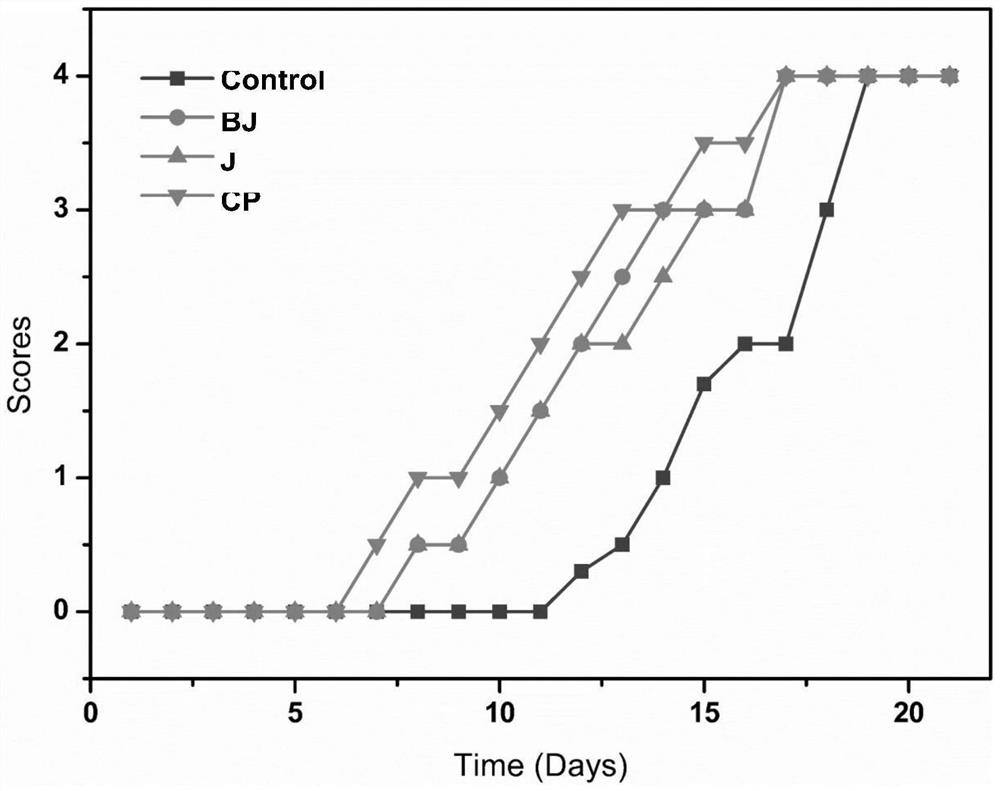
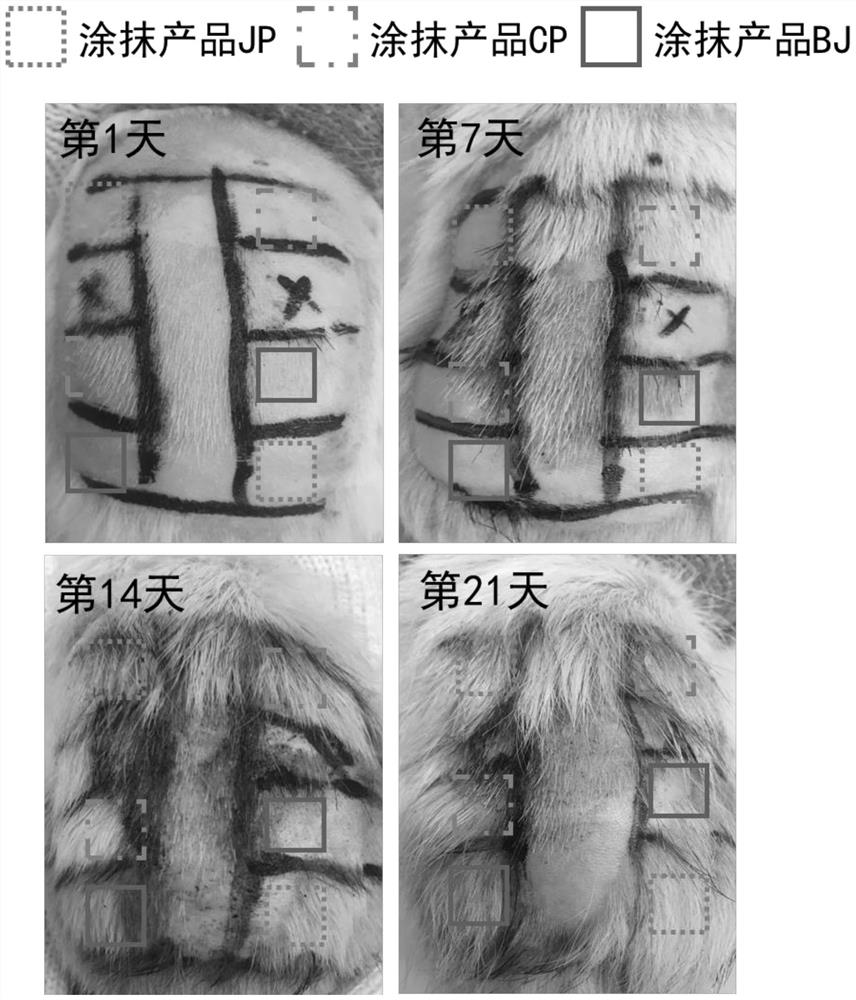
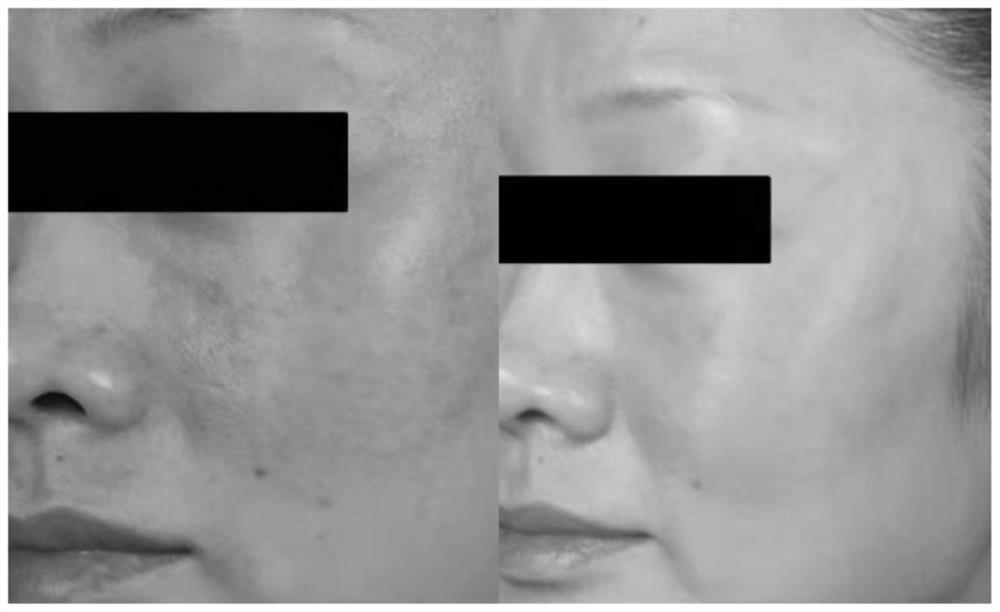
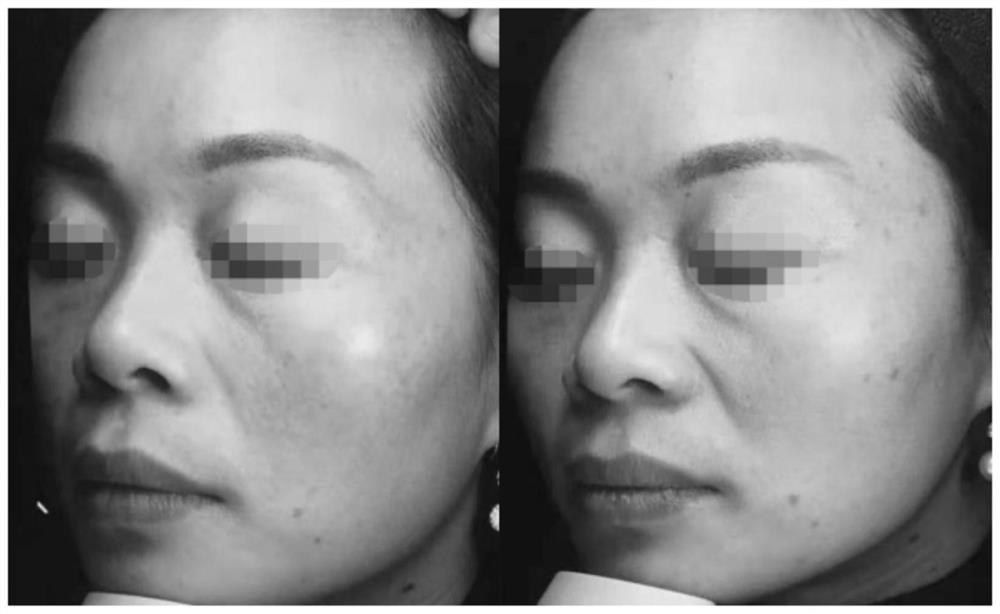
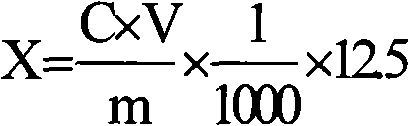Patents
Literature
63 results about "Monosodium dihydrogen phosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nutritional mineral fortification of milk
InactiveUS20030165597A1Preserve integrityEasy to correctMilk preparationFrozen sweetsPhosphatePotassium
A calcium and / or nutritional mineral fortified milk or milk powder product utilises pyrophosphates or orthophosphates in combination with maintenance of pH within the range of 6.5 to 7.5 to render the milk heat stable. Additional calcium and / or nutritional mineral is added in soluble form either before or after the phosphate addition. The preferred orthophosphates are one or more of monosodium dihydrogen orthophosphate, disodium hydrogen orthophosphate, trisodium orthophosphate, monopotassium dihydrogen orthophosphate, dipotassium hydrogen orthophosphate and tri potassium orthophosphate. Addition of an alkaline agent to adjust the pH is not needed if an appropriate mix of orthophosphates is used. The milk products or milk products recombined from milk powders are heat stable and do not have the problems of translucency, gritty mouth feel or sedimentation which can be associated with other stabilised fortified milks.
Owner:COMMONWEALTH SCI & IND RES ORG +1
Non-spherical polymer particles uniform in particle size as well as preparation method and application of non-spherical polymer particles
ActiveCN105832704AUniform particle sizeParticle size controllableSsRNA viruses negative-sensePeptide/protein ingredientsPolymer scienceBiological drugs
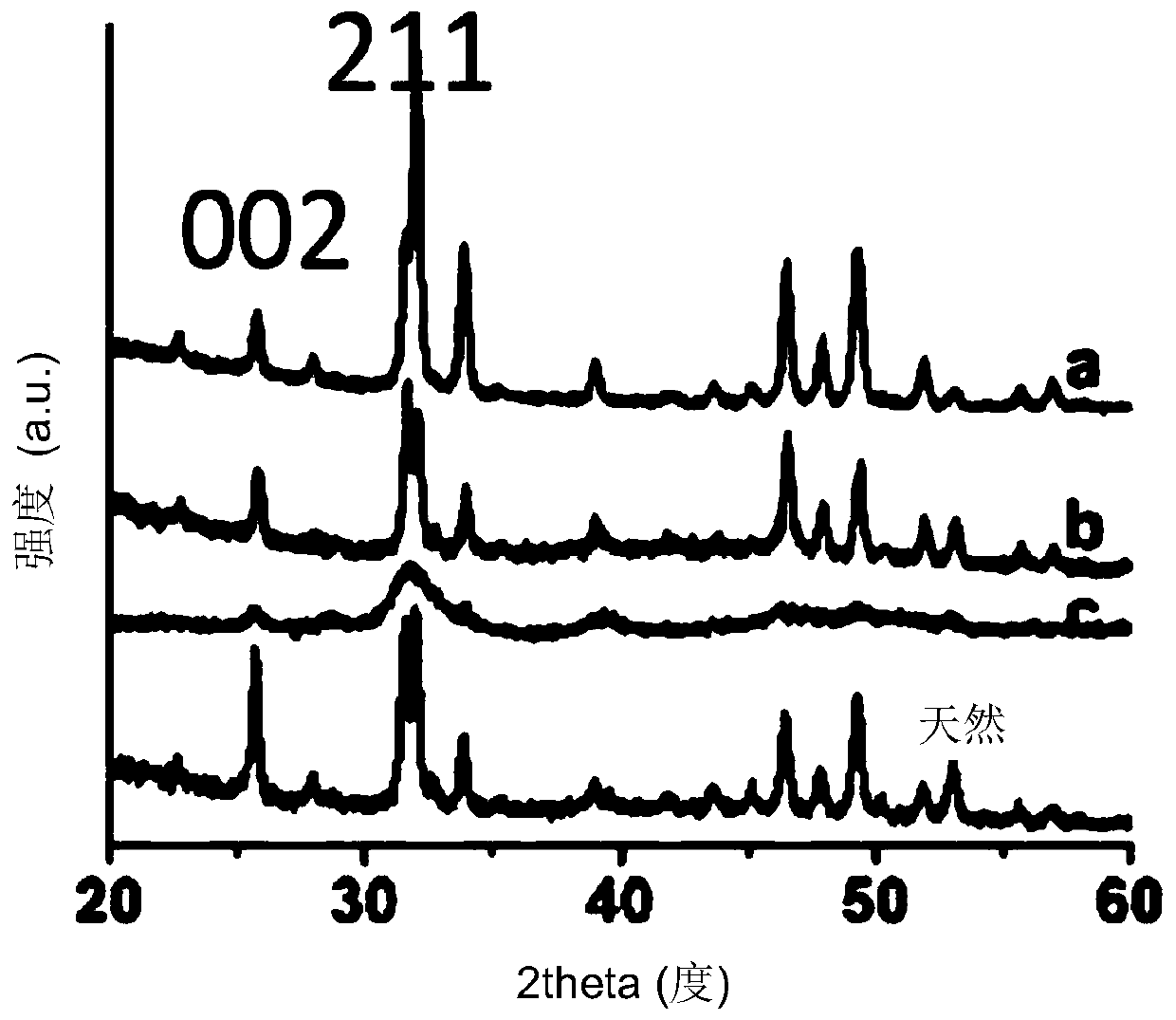
The invention provides non-spherical polymer particles uniform in particle size as well as a preparation method and an application of the non-spherical polymer particles. The non-spherical polymer particles are in the form of ellipsoids, short rods or fibers, and the interiors of the non-spherical polymer particles are of solid, hollow or porous structures; the short diameter of the non-spherical polymer particles ranges from 100nm to 30[micron]m, the long diameter of the non-spherical polymer particles is 1-60[micron]m, the ratio of the long diameter to the short diameter is 2-40, and the particle size distribution coefficient of the particles is less than 20%. The non-spherical polymer particles disclosed by the invention, which are uniform and controllable in particle size, are prepared by taking disodium hydrogen phosphate and / or sodium dihydrogen phosphate as a deformation inducing agent and by taking the influence of such conditions as the concentration of the disodium hydrogen phosphate and / or sodium dihydrogen phosphate, self-properties of a polymer, mass concentration of the polymer in an oil phase, the size of emulsion drops and the like into comprehensive consideration; the non-spherical polymer particles can be applied to various fields such as biological drug delivery, vaccine adjuvants, enzymatic catalysis, bio-separation, human tissue engineering field and the like; and the preparation method is simple to operate, mild in condition and easy for industrial enlarged production.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Colloidal gold method detecting reagent for extrauterine pregnancy and number of pregnancy days, and preparation thereof
InactiveCN101294966AResolve detectionSolve time-consuming and costly problemsBiological testingCelluloseObstetrics
The invention discloses an ectopic pregnancy and pregnant days colloid gold detection agent and manufacturing method thereof used for the problem of fast detection for pregnancy, comprising a piece of detecting test paper and urine sample treatment solution; wherein, the detecting test paper consists of a base plate, a cellulose nitrate membrane, a gold label anti-Beta HCG monoclone antibody layer, an Alpha-HCG monoclone antibody line, a sheep anti-rat polyclonal antibody line, an absorbing pad and a sample pad; the matching composition of the urine sample treatment solution is that: 12.5 to 18.0 portions of disodium hydrogen phosphate dodecahydrate, 1.2 to 1.7 portions of sodium dihydrogen phosphate dihydrate, 40.0 to 48.0 portions of sodium chloride and 4600 to 5300 portions of distilled water; the ectopic pregnancy and pregnant days colloid gold detection agent and manufacturing method of the invention are characterized by fast and simple detection, the operation of blood-drawing detection for 2 to 6 hours can be replaced by 2 to 3 minutes with time-saving, manual-saving and cost-saving; besides, the ectopic pregnancy and pregnant days colloid gold detection agent and manufacturing method thereof have the advantages of detecting ectopic pregnancy and pregnant days in a semiquantitative way, etc., and can be used for external detection of the pregnant condition and artificial abortion and assisted diagnosis.
Owner:崔学礼
Green-protecting and brittleness-keeping production process of cress
InactiveCN104137882AImprove qualityColor effectFruits/vegetable preservation by heatingMonosodium glutamateNutritive values
The invention discloses a green-protecting and brittleness-keeping production process of cress. The cress is prepared by mixing cress, salt, monosodium glutamate, a spice mixture, plant oil, sesame oil seasoning, citric acid, zinc acetate, calcium chloride, disodium hydrogen phosphate, sodium dihydrogen phosphate, hydrogen peroxide, guaiacol, nitric acid and hydrochloric acid according to a certain ratio. Equipment and instruments used in the production process comprise an ultraviolet and visible spectrophotometer, a texture analyzer, a color difference meter, a freezing centrifuge, a pulping machine, a pH meter and an electronic balance. By taking the corresponding technical measures, ideal green-protecting and brittleness-keeping effects can be achieved; by taking reasonable technical measures, various negative factors are effectively controlled, the nutritional value and sensory quality of a product are kept to the maximum extent, and healthy and delicious fruit and vegetable products are provided for customers.
Owner:TONGCHENG GUNIUBEI AGRI DEV
Active polysaccharide composite bone repair material
ActiveCN105749356AMeet the mechanical requirementsGood for crawlingTissue regenerationProsthesisHuman bodyCellulose
The invention discloses an active polysaccharide composite bone repair material which is good in biocompatibility, good in plasticity, certain in mechanical strength and moderate in degradation velocity in bodies and can meet clinical requirements. The active polysaccharide composite bone repair material comprises a bottle A and a bottle B, wherein the bottle A comprises powder of tetracalcium phosphate, calcium hydrophosphate, chitosan, monosodium phosphate and hydroxypropyl methylcellulose; the bottle B comprises mannitol, an MES solution and a rhBMP-2 biological active factor freeze-dried aquatic product. The active polysaccharide composite bone repair material can induce formation of new bone in a human body, can form active tissue, has the advantages of good compatibility, plasticity and the like, is free of toxic reaction and can be tightly connected with sclerotin after being cured.
Owner:ZHEJIANG RISING BIOTECH CO LTD
Preparation method of high-viscoelasticity injection composed of glucosamine and sodium hyaluronate
InactiveCN105982911AChange the high hygroscopicityReduce humidityOrganic active ingredientsInorganic non-active ingredientsDouble saltTherapeutic effect
The invention relates to a preparation method of a high-viscoelasticity injection composed of glucosamine and sodium hyaluronate. The injection mainly includes the components of glucosamine sulfate / sodium chloride double salt and the sodium hyaluronate with sodium dihydrogen phosphate and disodium hydrogen phosphate, as auxiliary materials, being used as a pH regulator. Sodium chloride is employed as an osmotic pressure regulator. The injection is greatly reduced in hygroscopicity and enhanced in flowability, is improved in stability, has good treatment effects, low drug resistance rate and less adverse reaction, and can be used in intra-articular injection therapy on arthritis.
Owner:上海建华精细生物制品有限公司
Compound glycyrrhizin lyophilized powder injection and preparation method thereof
InactiveCN103860483APowder deliveryOrganic active ingredientsDipotassium hydrogen phosphateLiquid content
The present invention relates to a compound glycyrrhizin lyophilized powder injection and a preparation method thereof, particularly to compound glycyrrhizin for injection, a compound monoammonium glycyrrhizinate S lyophilized powder injection for injection, and a preparation method thereof. According to the present invention, the prescription contains a lyophilized powder injection comprising glycyrrhizin (monoammonium glycyrrhizinate) adopted as an active component, glycine, cysteine hydrochloride and a disodium hydrogen phosphate-sodium dihydrogen phosphate or dipotassium hydrogen phosphate-potassium dihydrogen phosphate pH buffer, and a preparation method of the lyophilized powder injection; and the drug liquid content is stable during the compound glycyrrhizin lyophilized powder injection preparation process, the finished product can be stored at a room temperature, the injection stability is significantly improved, the safety and the effectiveness of the injection are well ensured, and the warehousing transportation cost during the injection production storage and transportation process is effectively reduced.
Owner:北京藏卫信康医药研发有限公司
Recombinant bovine alkaline fibroblast growth factor eye drops
ActiveCN104606666AReduce usageLess irritatingSenses disorderPeptide/protein ingredientsPolyvinyl alcoholHuman albumin
The invention discloses recombinant bovine alkaline fibroblast growth factor eye drops and a preparation method thereof. The recombinant bovine alkaline fibroblast growth factor eye drops are prepared from the following components by weight: 1500-2500 IU of recombinant bovine alkaline fibroblast growth factor, 0.04-0.12mg of human albumin, 2.0-6.0mg of polyvinyl alcohol, 4.0-6.0mg of sodium chloride, 0.20-0.30mg of disodium hydrogen phosphate, 0.40-0.50mg of sodium dihydrogen phosphate and 0.4ml of water for injection. According to the recombinant bovine alkaline fibroblast growth factor eye drops disclosed by the invention, the dosage of auxiliary material is reduced in the eye drops, the stability of the eye drops is also ensured, the shelf life of the eye drops is prolonged, and the application range is enlarged; without the addition of a bacteriostatic agent, the potential risk caused by the bacteriostatic agent and the toxic or side effect to eyes are prevented, and the irritation of the eye drops to eyes is reduced, and the eye drops are safe in use and high in compliance of a patient.
Owner:ZHUHAI ESSEX BIO PHARMA
Human albumin nano-particle and preparation method thereof
The invention discloses a nano-particle utilizing human albumin as a carrier material. The nano-particle comprises 50g of a drug, 30-80g of lecithin, 2-16g of cholesterol, anhydrous ethanol, 20-120g of disodium hydrogen phosphate, 20-120g of sodium dihydrogen phosphate, 100-200g of human albumin (calculated based on its solution), 50-150g of Arabic gum and 1-100ml of hydrochloric acid.
Owner:国药集团贵州血液制品有限公司
Povidone-iodine solution with enhanced stability and preparation method thereof
ActiveCN112315974AGuaranteed stabilityMaintain acid-base balanceAntibacterial agentsAntimycoticsPhosphateSodium sulfate
The invention discloses a povidone-iodine solution with enhanced stability and a preparation method thereof. The povidone-iodine solution consists of povidone iodine, sodium dihydrogen phosphate, disodium hydrogen phosphate dodecahydrate, potassium iodate, lauryl sodium sulfate and purified water, and the concentrations of the raw materials are as follows: 5-20 g / L of povidone iodine, 1-10 g / L ofsodium dihydrogen phosphate, 0.5-2 g / L of disodium hydrogen phosphate dodecahydrate, 0.2-1.0 g / L of potassium iodate, and 1-10 g / L of lauryl sodium sulfate, and the pH of a sodium hydroxide solution is adjusted to 5.20-5.30. The sodium dihydrogen phosphate and the disodium hydrogen phosphate dodecahydrate are used as a pH value adjusting buffer pair, so that the acid-base balance of the solution can be maintained, and a buffer effect can be achieved. The potassium iodate and the lauryl sodium sulfate are used as pH stabilizers, the sodium hydroxide is used as a pH regulator, and the pH value of the povidone-iodine solution can be controlled within a specified range, so that the stability of the effective iodine content is ensured, and the bactericidal efficacy of the povidone-iodine solution is maintained.
Owner:乐泰药业有限公司
Culture medium for enriching salmonella, shigella and staphylococcus aureus in composite way and preparation method thereof
InactiveCN102827795AShorten enrichment timeBacteriaMicroorganism based processesBiotechnologyLithium chloride
The invention discloses a culture medium for enriching salmonella, shigella and staphylococcus aureus in a composite way and a preparation method thereof. The culture medium comprises 13-16 parts of tryptone, 4-7 parts of soy peptone, 2-3 parts of monosodium orthophosphate, 2-3 parts of glucose, 1,000 parts of distilled water, 0.07-0.13 part of bile salt, 25-40 parts of sodium chloride, 0.4-1 part of lithium chloride, 1.5-3.5 parts of mannite and 0.0002-0.0004 part of potassium tellurite, and the pH of the culture medium is 7.1-7.3. The culture medium can be used for restraining the growth of other pathogenic microorganisms while enriching three target pathogenic bacteria simultaneously, can be directly applied to separate culturing of target bacteria and biological assay experiments, and can be directly applied to a detection technology for a plurality of pathogenic bacteria based on one detection platform such as multiple PCRs (Polymerase Chain Reactions) and the like for making a diagnosis report.
Owner:SOUTH CHINA UNIV OF TECH
Composite modifier for bread
InactiveCN105192016AEasy to processPrevent agingDough treatmentPre-baking dough treatmentBiotechnologyCellulose
The invention discloses a composite modifier for bread, comprising the following components: sodium alginate, potato starch, citric acid, vitamin C, calcium lactate stearate, glycerin monostearate, carboxymethylcellulose, sodium dihydrogen phosphate, disodium hydrogen phosphate, salt, psyllium seed gum, okra gum and a compound enzyme. The composite modifier for bread dislcosed by the invention is good in mixing performance, high in safety, convenient to use, abundant in nutrition, and capable of effectively improving processability of bread in the preparation process, thus enabling the flavor and taste of the bread to be obviously perfected and kept for a long term, and prolonging the shelf life of bread.
Owner:JINAN SHUNXIANG MEDICINE SCI & TECH
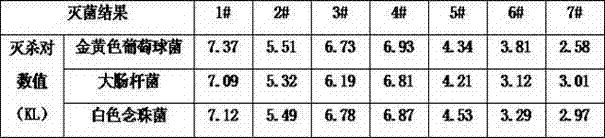
Lysostaphic complex enzyme oral cavity disinfectant and preparation method thereof
InactiveCN104524559AQuick killWon't hurtAntibacterial agentsOrganic active ingredientsOral diseaseChlorhexidine Acetate
The invention discloses a lysostaphic complex enzyme oral cavity disinfectant and a preparation method thereof. The lysostaphic complex enzyme oral cavity disinfectant comprises the following components in parts by mass: 20-40 parts of lysostaphin, 21-41 parts of antibacterial peptide, 21-41 parts of chlorhexidine acetate, 31-42 parts of sorbitol, 21-31 parts of sodium dihydrogen phosphate and 21-30 parts of disodium hydrogen phosphate. The oral cavity disinfectant can be used for treating oral cavity diseases, has the advantages of remarkable curative effect and no alcohol and drug resistance. The invention further discloses a preparation method of the oral cavity disinfectant. The preparation method comprises the following steps: 1) dissolving the lysostaphin, the ntibacterial peptide and the hlorhexidine acetate into distilled water, stirring to completely dissolve the lysostaphin, the ntibacterial peptide and the hlorhexidine acetate; 2) dissolving sorbitol into distilled water, and stirring to completely dissolve the sorbitol; 3) dissolving the sodium dihydrogen phosphate and the disodium hydrogen phosphate into distilled water, and stirring to completely dissolve the sodium dihydrogen phosphate and the disodium hydrogen phosphate; and 4) uniformly mixing solutions obtained in the steps 1), 2) and 3), and then filling the mixed solution. The preparation method has the advantages of simple process and strong operability.
Owner:杨陈
Composite additive of quick-freezing wonton wrappers
InactiveCN105192486AImprove toughnessImprove water locking abilityFood preparationFood ingredient as mouthfeel improving agentBiotechnologyCellulose
The invention discloses a composite additive of quick-freezing wonton wrappers. The composite additive is prepared from sodium alginate, citric acid, vitamin C, sodium dihydrogen phosphate, disodium hydrogen phosphate, carboxymethylcellulose, table salt, edible alkali and cassia seed gelatin. By virtue of adopting the composite additive of the quick-freezing wonton wrappers, the problems that quick-freezing wontons are easy to crack, the appearance color and cluster and the mouth feel become poor and the quick-freezing wontons cannot be boiled for a long period in water are solved; the produced wonton wrappers have a good mouth feel and abundant nutrients. The composite additive of the quick-freezing wonton wrappers, disclosed by the invention, has good solubility, good mixing property, high safety, simplicity and convenience in utilization and abundant nutrients; and the problems that the wrappers are thin and easy to crack and have a dark color in processing, boiling and freeze thawing processes of the wonton wrappers can be improved.
Owner:JINAN SHUNXIANG MEDICINE SCI & TECH
Method for preparing freeze dried powder injection of rhodiola root
The invention relates to a rhodiola root powder and injection preparation and its preparing method, wherein the active component of the medicament is salidroside, the preferred supporting agent is one of mannitol, dextran, glucose, sorbitol, lactose, gelatin, disodium hydrogen phosphate - sodium dihydrogen phosphate, calcium lactobionate, dextran- glucose.The ratio of the rhodiola root extract and the supporting agent is 1 : 0.2-1.2.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Composition and preparation method of deslanoside injection
InactiveCN112336687AImprove the level ofQuality improvementOrganic active ingredientsInorganic non-active ingredientsGlycerolSterility
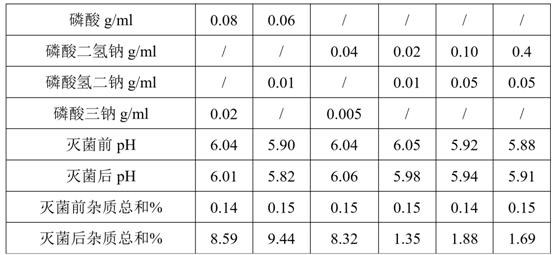
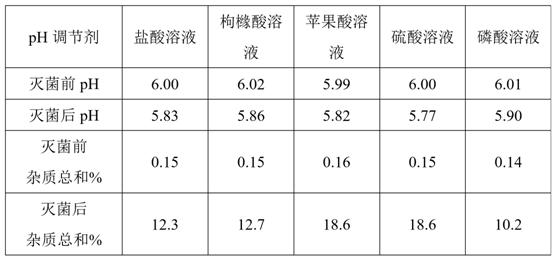
The invention discloses a composition and preparation method of a deslanoside injection. According to formulation composition, the composition consists of deslanoside, ethanol, glycerin, sodium dihydrogen phosphate, disodium hydrogen phosphate and water for injection,wherein the use amount of the sodium dihydrogen phosphate is 0.02-0.8 g / ml, and the use amount of the disodium hydrogen phosphate is0.01-0.1 g / ml. The deslanoside injection disclosed by the invention is stable in quality, can tolerable hot pressurized sterilization, and F0 value is not less than 12; the sterility assurance levelis high, the process of the deslanoside injection is simple and efficient and the quality is controllable.
Owner:南京科宁检测科技有限公司
Lysostaphin compound enzyme disinfectant and preparation method thereof
InactiveCN104721812AImprove disinfection efficiencyNo side effectsAntibacterial agentsOrganic active ingredientsBiotechnologyChlorhexidine Acetate
The invention discloses a lysostaphin compound enzyme disinfectant comprising the following components in parts by weight: 1.1-10 parts of lysostaphin, 1.1-10 parts of antimicrobial peptide, 1.1-10 parts of chlorhexidine acetate, 10-20 parts of sorbitol, 1.1-10 parts of sodium dihydrogen phosphate and 1.1-10 parts of disodium hydrogen phosphate. The lysostaphin compound enzyme disinfectant has the advantages of high disinfection efficiency, good disinfection effect, easy storage and stable performance. The invention further discloses a preparation method of the lysostaphin compound enzyme disinfectant. The preparation method mainly comprises the following steps: 1) dissolving the lysostaphin, the antimicrobial peptide and the chlorhexidine acetate in water, mixing the solutions, and shaking uniformly; 2) adding sorbitol into the solution obtained in the step 1), and shaking uniformly; and 3) dissolving the sodium dihydrogen phosphate and the disodium hydrogen phosphate in distilled water, stirring uniformly, adding the obtained solution into the solution obtained in the step 2), and shaking uniformly to obtain the lysostaphin compound enzyme disinfectant. The preparation method has the advantages of simple process and strong operability.
Owner:杨陈
Cool pillow and manufacturing method thereof
InactiveCN103005976AExtended low temperature timeHas the effect of dissolving and absorbing heatPillowsHeat-exchange elementsPhysical chemistryP phosphate
The invention discloses a cool pillow and manufacturing method. The content of the cool pillow comprises the following components: 15-60% of urea, 10-30% of phosphate, 5-15% of guar gum and 25-70% of drinking water, wherein the guar gum is cation-type or anion-type guar gum, the concentration of the urea is 33-50% and the phosphate is disodium hydrogen phosphate and sodium dihydrogen phosphate. The cool pillow is manufactured through the following steps in sequence: firstly dissolving alkaline refrigerants, i.e. the urea and the disodium hydrogen phosphate in drinking water, wherein the solution is alkaline; then adding the guar gum into the solution and stirring uniformly; when high molecular colloidal sol is formed, adding the acidic refrigerant sodium dihydrogen phosphate into the mixture, mixing uniformly to obtain the content; and finally packaging the content into a packaging bag, thereby obtaining the cool pillow. The normal use time of the cool pillow is longer than that of a common cool pillow. The phosphate in the formula is dissolved at the temperature of 27 DEG C so as to absorb heat, so that the cool pillow can be maintained in a low temperature state for a longer period of time before reaching 30-DEG C .
Owner:湖州新驰医药科技有限公司
Papaverine hydrochloride pharmaceutical composition for injection and preparation method
The invention relates to a papaverine hydrochloride pharmaceutical composition for injection and a preparation method. Specifically, the pharmaceutical composition provided by the invention comprisespapaverine hydrochloride and pharmaceutic adjuvants, wherein the pharmaceutic adjuvant comprises mannitol and edetate, and the weight ratio of the papaverine to the mannitol to the edetate is 30: (15-500): (2-10); the pharmaceutical composition further comprises an acid-base regulator, and the dosage of the acid-base regulator is that when the freeze-dried powder injection is dissolved into a solution containing papaverine hydrochloride with the concentration of 15mg / ml by using water for injection, the pH value of the solution is within the range of 2.5-4.0, the acid-base regulator is selected from sodium hydroxide, potassium hydroxide, sodium dihydrogen phosphate, disodium hydrogen phosphate, monopotassium phosphate, dipotassium phosphate, hydrochloric acid, phosphoric acid, nitric acid,sulfuric acid or a combination thereof, especially a 1M hydrochloric acid solution or a 1M sodium hydroxide solution. The invention also provides a preparation method of the papaverine hydrochloridepharmaceutical composition for injection. The papaverine hydrochloride pharmaceutical composition for injection is prepared by a method for freeze-drying powder injection. The papaverine hydrochloridepharmaceutical composition disclosed by the invention has excellent technical effects as described in the specification.
Owner:山东北大高科华泰制药有限公司
Method for measuring contents of cationic surface active substances by bromothymol blue spectrometry
InactiveCN101576480ASignificant progressSignificant positive effectColor/spectral properties measurementsBromothymol blueAlkaline earth metal
The invention provides a method for measuring contents of cationic surface active substances in solid alkali metal salts, alkaline-earth metal salts or alkaline-earth metal hydroxides by a bromothymol blue spectrometry, which comprises the following steps: using nitric acid to dissolve a sample to be measured to prepare solution of alkali metal or alkaline-earth metal with ion concentration of 0.1 to 0.5 mol / L; adding bromothymol blue solution into the prepared solution, adjusting the pH of the solution to between 7.6 and 7.8 by using monosodium orthophosphate and disodium hydrogen phosphate buffer solution, allowing the solution to stand for 20 to 26 hours, and measuring the absorbency of the sample solution; after the absorbency A of the sample solution is deducted from blank absorbency Ao, forming a linear relation with the content of a complex compound formed by the reaction of the cationic surface active substances and the bromothymol blue in the sample solution; and obtaining the contents of the cationic surface active substances in the sample solution to be measured through a standard curve, and then obtaining the contents of the cationic surface active substances in the solid alkali metal and the alkaline-earth metal salts through conversion. The analysis process is simple and quick, the analysis result accuracy is high, and the repeatability is good.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Method for synthesizing phytosterol ester in reverse micelle enzyme system
The invention discloses a method for synthesizing phytosterol ester in a reverse micelle enzyme system based on lipase interface activation characteristics. The specific synthesis method comprises thefollowing steps: constructing a reverse micelle enzyme system, wherein a non-immobilized water-soluble lipase buffer aqueous solution (50 mmol / mL sodium dihydrogen phosphate-disodium hydrogen phosphate, pH 6.5-8) into an isooctane solution of 2-ethylhexyl succinate sodium sulfonate (AOT), a clarified reverse micelle enzyme system is obtained through energy-gathered ultrasound, and a ratio of [enzyme] (mg / mL) to [water] (mmol / mL) to [AOT] (mmol / mL) is (3-8):(200-600):(20-30); adding 50-100 mmol / mL phytosterol and C12-C18 fatty acid accounting for 2-4 times the molar mass of the phytosterol into the reverse micelle enzyme system, and carrying out a reaction for 3-6 hours at a temperature of 35-55 DEGC under an enzyme loading capacity accounting for 1.5-3% of the total substrate, wherein theprocess is assisted by divergent ultrasonic action, and the yield of the esterification product measured by an HPLC method is 85.79-92.82%; and purifying the product by a solvent crystallization method, wherein the purity is 98.56-99.43% measured by the HPLC method. The method has the advantage of high efficiency and high yield production of phytosterol ester.
Owner:NANCHANG UNIV
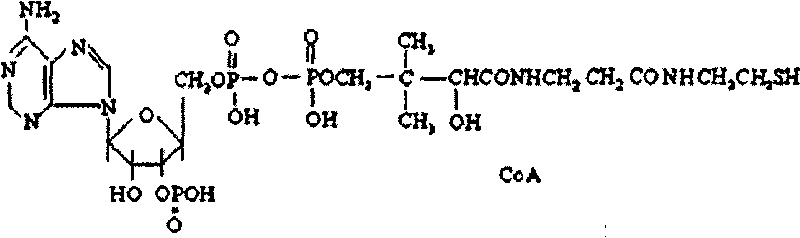
Coenzyme A medicament freeze-drying preparation and process for preparing same
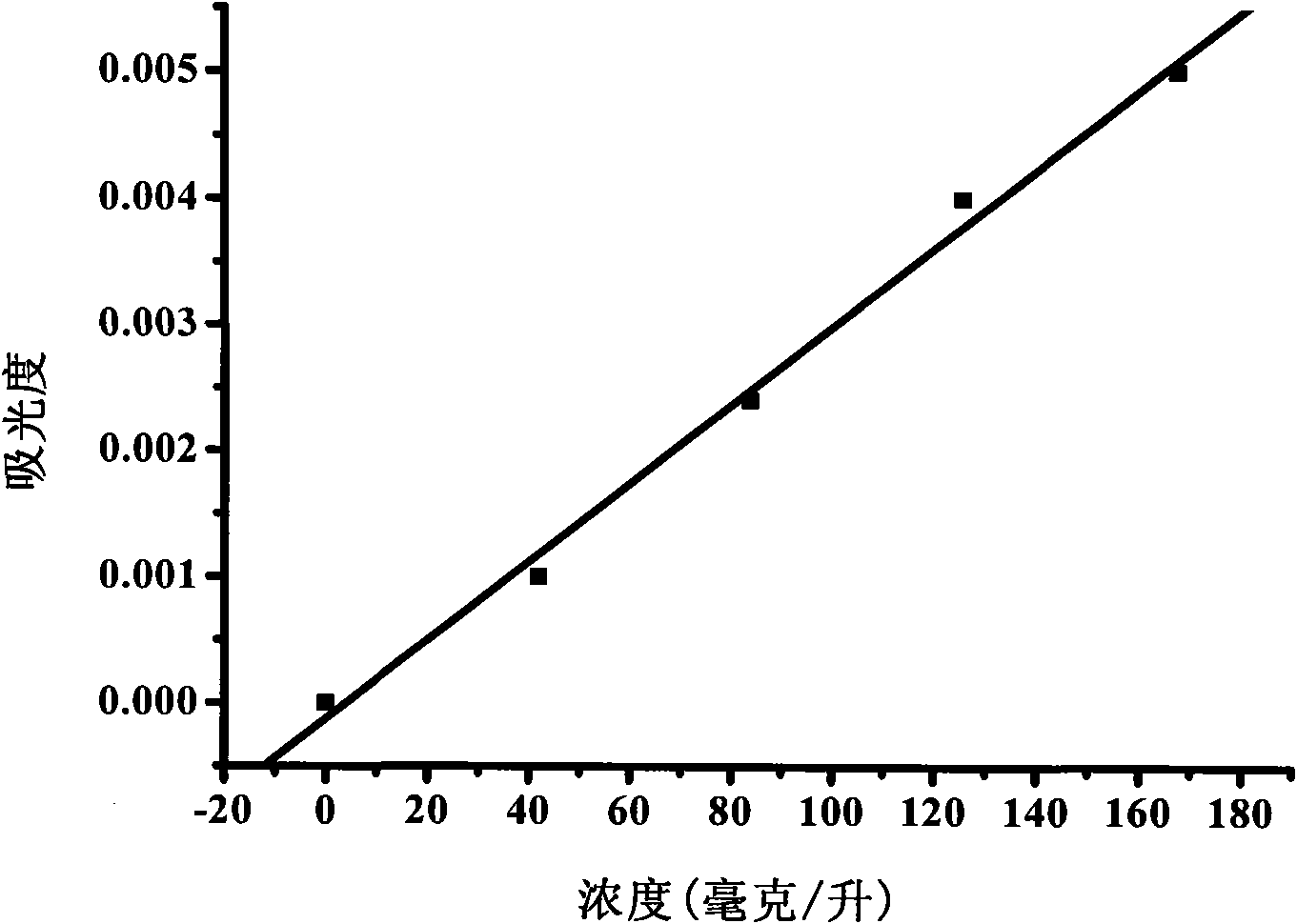
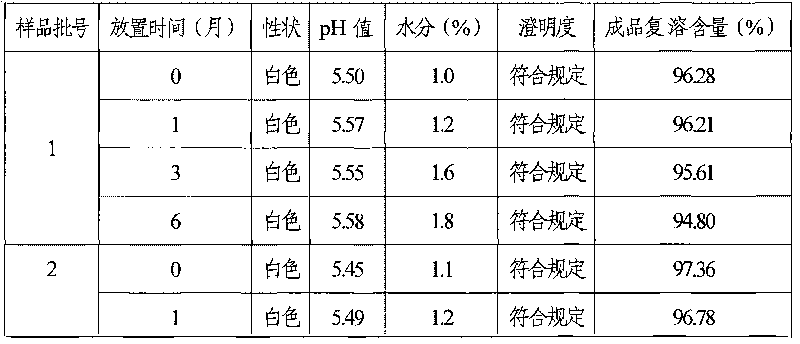
ActiveCN101693015AGuaranteed stabilityNot easy to degradePowder deliveryOrganic active ingredientsPhosphoric acidFreeze dry
The invention discloses a coenzyme A medicament freeze-drying preparation, which adopts disodium hydrogen phosphate and sodium dishydrogen phosphate buffered salt liquor to protect a coenzyme A in the process of preparing the freeze-drying preparation. The freeze-drying preparation selects phosphate buffered salt liquor in the process of preparing medical liquor to enable the coenzyme A to be in the liquor with relatively constant pH values. Simultaneously, the phosphate buffered salt liquor is favorable for stabilizing a coenzyme A molecular structure containing phosphate groups, a coenzyme A potency (marked content) is lowered by no more than 2.5% from the process of preparing and filling to the beginning of freeze-drying.
Owner:MAANSHAN BBCA PHARMA
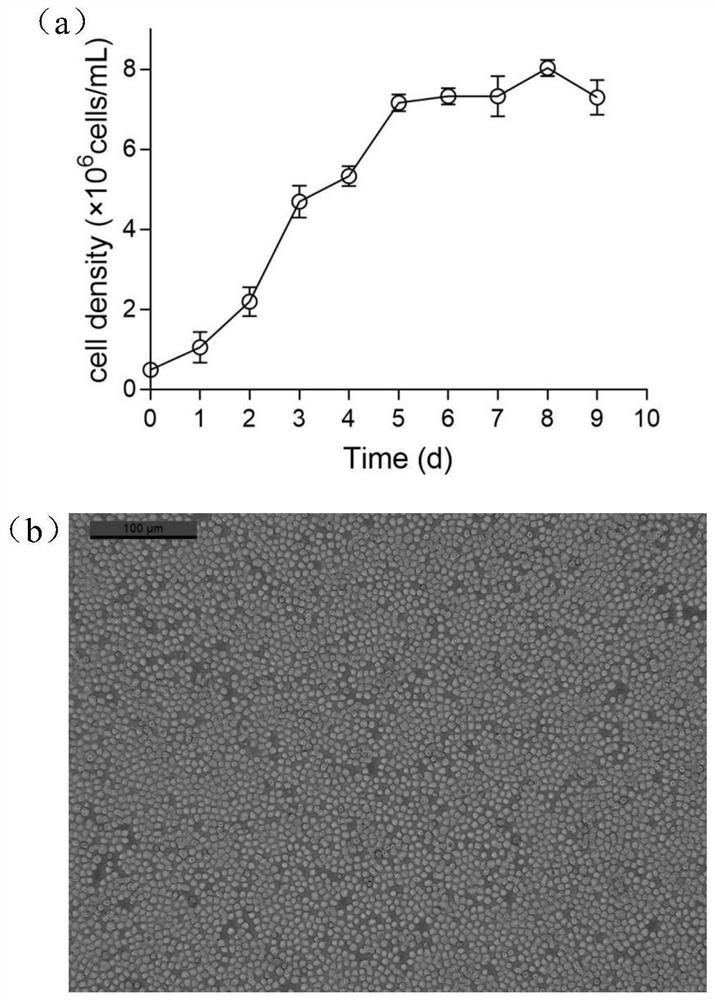
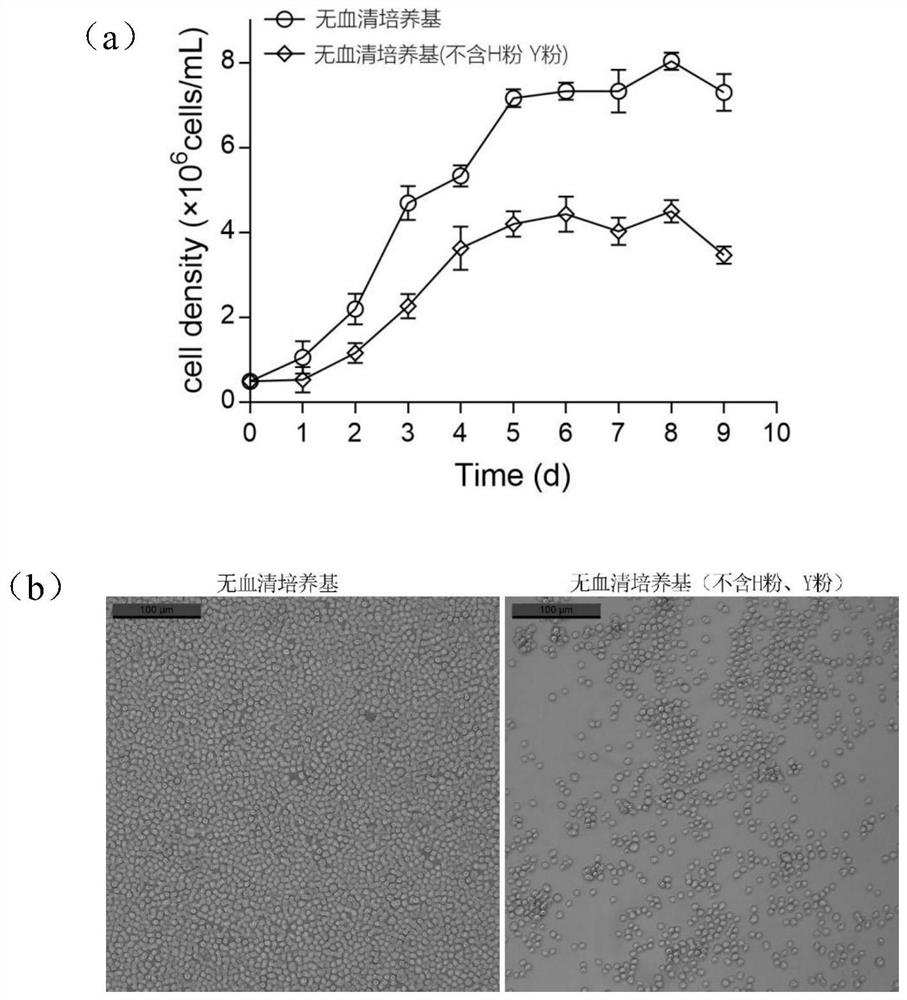
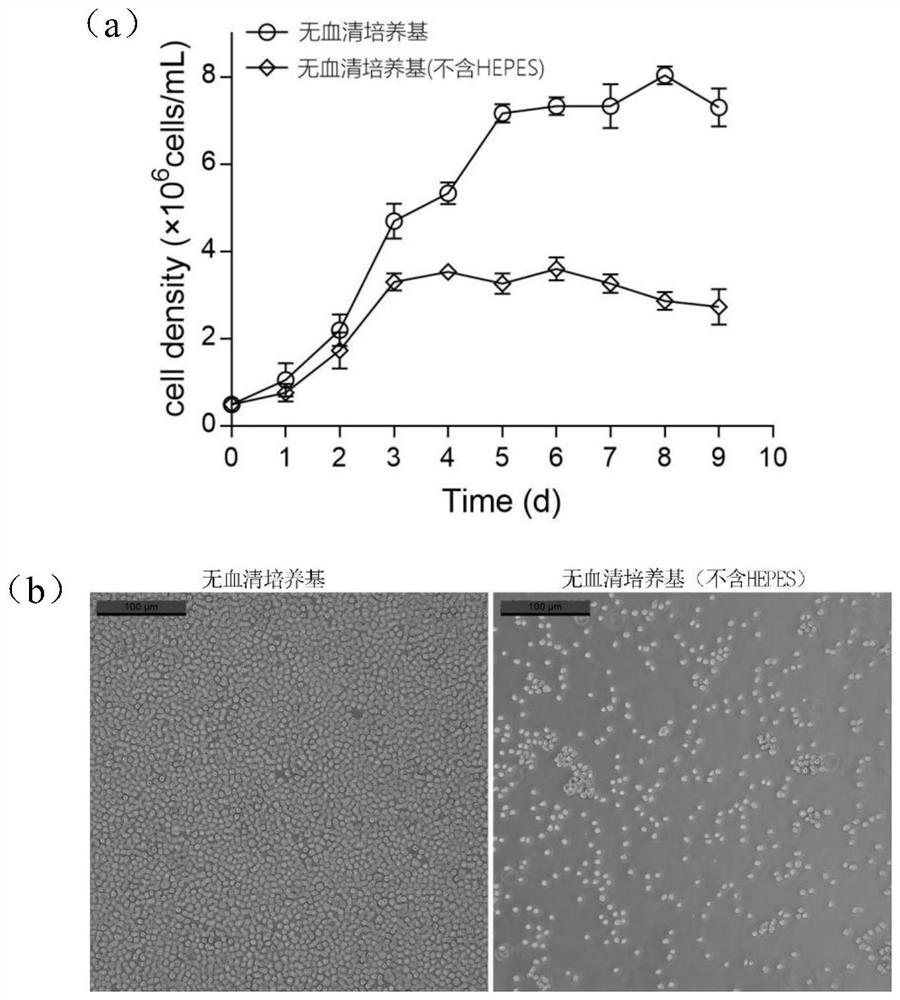
Serum-free culture medium suitable for large-scale suspension multiplication culture of CHO cells and preparation and application of serum-free culture medium
PendingCN113913368AAvoid influenceImprove long-term stabilityCulture processArtificial cell constructsSodium bicarbonateTrace element composition
The invention discloses a serum-free culture medium, a preparation method thereof and an application of the serum-free culture medium to large-scale suspension multiplication culture of CHO cells. The pH value of the serum-free culture medium is 6.9-7.0, and the serum-free culture medium comprises an amino acid composition, an inorganic salt composition, a vitamin composition, a trace element composition, a buffer system composition, a supplementary factor composition and water. On the basis of the serum-free culture medium, the amino acid composition comprises 1-2 mg / L of glutamine dipeptide GlutaMAX-I, the buffer system composition comprises 3-8 g / L of HEPES, 1-5 g / L of sodium bicarbonate, 100-300 mg / L of sodium dihydrogen phosphate, 100-300 mg / L of disodium hydrogen phosphate, 3-10 g / L of sodium chloride and 200-500 mg / L of potassium chloride, and the supplementary factor composition comprises 10-30 g / L of a soybean extract, 10-30 g / L of a yeast extract and 80-100 [mu]g / L of Long-R3-IGF-I. The preparation method comprises the following steps: weighing and mixing the components according to the formula, adjusting the pH value to 6.9-7.0, and performing filtering by using a filter membrane with the pore diameter of 0.22 [mu]m.
Owner:TONGDE HOSPITAL OF ZHEJIANG PROVINCE
Mixture of quinoa peptide and soybean peptide
InactiveCN110786519AIncrease profitChange molecular structureProtein composition from vegetable seedsVegetable proteins working-upBiotechnologyAmylase
The invention discloses a mixture of quinoa peptide and soybean peptide. The mixture comprises the following raw materials in parts by weight: 30-50 parts of quinoa, 80-150 parts of purified water, 20-30 parts of petroleum ether, 130-140 parts of deionized water, 15-20 parts of alpha-amylase, 8-12 parts of glucase I, 8-12 parts of glycolase II, 8-12 parts of protease, 30-50 parts of soybeans, 6-12parts of disodium hydrogen phosphate, 6-12 parts of sodium dihydrogen phosphate, 6-12 parts of basic protease, 6-12 parts of flavor protease and 6-12 parts of tyrosine. The invention relates to the technical field of preparation of the mixture of the quinoa peptide and the soybean peptide. The mixture of the quinoa peptide and the soybean peptide is capable of maintaining activity of collagen peptide to the maximum extent and reducing loss of nutrients, protein peptide after freeze drying is very stable, the preservation time of the soybean protein can be prolonged, the needs of modern peoplefor protein can be met, the mixture is practical, and the prepared product can be of diversified forms, can be used in solid beverages or oral liquids, and can also be added to functional foods.
Owner:广东羲准生物科技有限公司
Atropine sulfate eye drops
InactiveCN111840221AReduce stimulationLow costSenses disorderInorganic non-active ingredientsAtropine sulfateBiochemistry
The invention relates to atropine sulfate eye drops, which comprise the following raw materials: atropine sulfate, sodium chloride, disodium hydrogen phosphate, sodium dihydrogen phosphate and water,the pH value of the atropine sulfate eye drops is 4.5-5.5, and the concentration of atropine sulfate is 0.09-0.11 mg / ml. The product has the advantages of simple formula, good stability, no stimulation to human eyes and simple preparation process, and is beneficial to long-term use.
Owner:安徽博诺美科生物医药有限公司
Polyphosphate capable of regulating and controlling hair growth and preparation method thereof
ActiveCN113730436APromote growthGood effectCosmetic preparationsHair cosmeticsBiotechnologyHydrogen phosphate
The invention provides polyphosphate capable of regulating and controlling hair growth and a preparation method thereof. The polyphosphate comprises dihydric phosphate and dibasic hydrogen phosphate: the dihydric phosphate comprises one or more of sodium dihydrogen phosphate, monopotassium phosphate and monocalcium phosphate, and the total proportion of the dihydric phosphate is 20-100%; and the dibasic hydrogen phosphate comprises one or more of disodium hydrogen phosphate, dipotassium phosphate and dicalcium phosphate, and the total proportion of the dibasic hydrogen phosphate is 0-80%. The preparation process comprises the following steps: carrying out preheating treatment at 400-500 DEG C, heating to 1000-1300 DEG C, carrying out heat preservation treatment, finally carrying out quenching treatment to obtain the product, and performing ball milling according to the use performance of the material to obtain samples with different sizes. The invention overcomes the defects of the prior art, has an important effect of regulating and controlling hair growth through specific proportion matching of the dihydric phosphate and the sodium dihydrogen phosphate, and is suitable for anti-hair loss and hair growth products.
Owner:北京普世安生物科技有限公司
Composite solution with combined application of recombinant III-type humanized collagen and hyaluronic acid and preparation process of composite solution
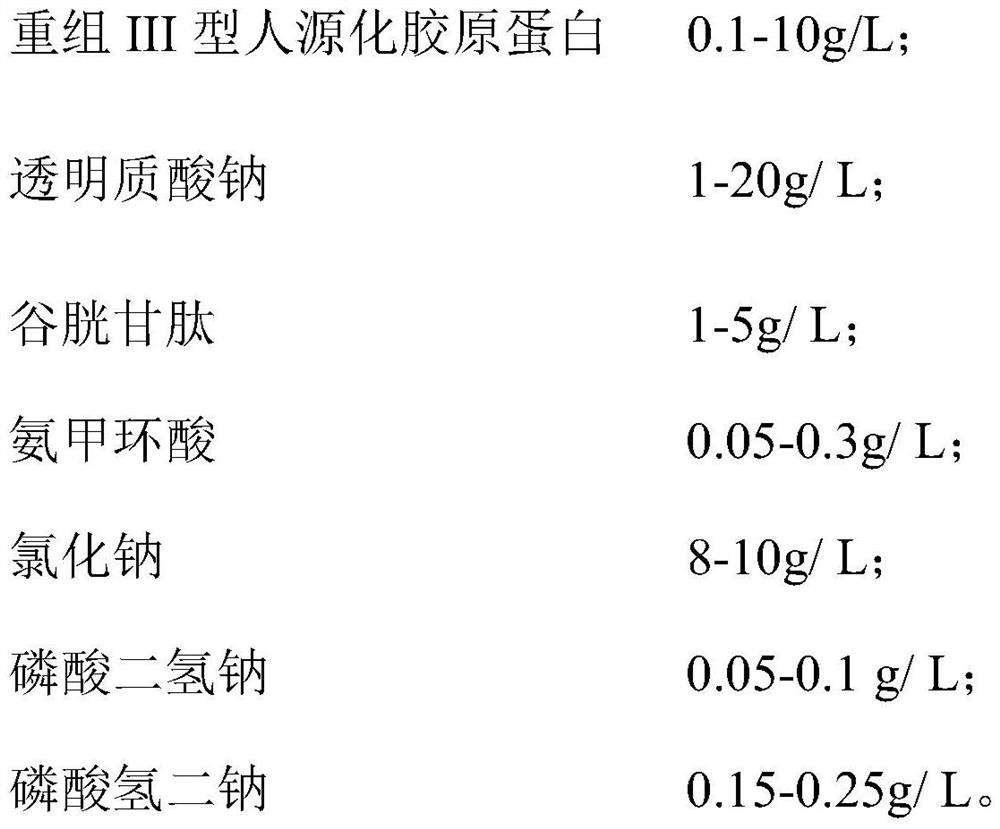
PendingCN114796011AReactiveAnti-inflammatoryCosmetic preparationsToilet preparationsAbsorption (skin)Tranexamic acid
The invention discloses a composite solution with combined application of recombinant III-type humanized collagen and hyaluronic acid and a preparation process of the composite solution, and relates to the technical field of medical cosmetology. A physiological buffer solution is used as a solvent, and comprises the following components by concentration: 0.1-10g / L of recombinant III-type humanized collagen; 1 to 20 g / L of sodium hyaluronate; 1-5 g / L of glutathione; the concentration of tranexamic acid is 0.05 to 0.3 g / L; 8-10g / L of sodium chloride; sodium dihydrogen phosphate is 0.05 to 0.1 g / L; and 0.15 to 0.25 g / L of disodium hydrogen phosphate. According to the composite solution combining the recombinant III-type humanized collagen and the hyaluronic acid, the sodium hyaluronate is used as a filling support, the water absorption and moisturizing performance is excellent, the moisturizing and anti-wrinkle effects are achieved immediately after injection, and the preparation method is simple and easy to operate. Then the occupation effect of the recombinant III-type humanized collagen, glutathione and other substances stimulates the body to synthesize collagen, and tranexamic acid has the effects of resisting allergic reaction and diminishing inflammation, so that the problems of moisturizing and wrinkle resistance of the skin are fundamentally solved.
Owner:湖南精准医疗器械科技有限公司
Preparation method of oral high-density lipoprotein liposome
InactiveCN103860470APeptide/protein ingredientsMacromolecular non-active ingredientsA lipoproteinPolyvinyl alcohol
The invention provides a preparation method of oral high-density lipoprotein liposome. The oral high-density lipoprotein is prepared from the components (calculated according to 1000ml) of: 20-400 ml of high-density lipoprotein solution, 20-150g of sorbitol, 2-50g of beta-cyclodextrin, 2-50g of PEG (Polyethylene Glycol) (2000-8000), 2-15g of disodium hydrogen phosphate (12 H2O), 0.2-2g of disodium hydrogen phosphate (2 H2O), 2-60g of polyvinyl alcohol, 15-90g of soybean phospholipid, 2-20g of vitamin E, 0.5-20g of cholesterol and suitable amount of ethyl alcohol. The prepared oral high-density lipoprotein liposome has the characteristics that the oral high-density lipoprotein liposome can tolerate the conventional moist heat sterilization, can also keep the stability for more than 2-5 hours in the strong acid environment with a pH value smaller than 2 and is also insensitive to the attenuation treatment, and the stability of the oral high-density lipoprotein liposome is greatly improved compared with that of the conventional liposome.
Owner:国药集团贵州血液制品有限公司
Oral care composition
ActiveCN110769801AOrganic active ingredientsCosmetic preparationsNeutral Amino AcidsDipotassium hydrogen phosphate
An oral care composition is disclosed comprising from 0.01 to 50% by weight of a calcium source, a water soluble phosphate source, a water soluble silicate source and from 0.01 5 to 10% by weight of an amino acid or its physiologically acceptable salt or a mixture thereof, wherein the calcium source is calcium hydroxide, calcium oxide, calcium glycerophosphate, calcium lactate, calcium sulfate, calcium salts of citric acid, calcium chloride, calcium nitrate, calcium acetate, calcium gluconate, calcium formate, calcium malate, calcium propionate, calcium butyrate, calcium bicarbonate, monocalcium phosphate anhydrous, dicalcium phosphate anhydrous, tricalcium phosphate, octacalcium phosphate, calcium silicate, calcium carboxymethyl cellulose, calcium alginate, calcium carbonate or mixtures thereof; wherein the phosphate source is trisodium phosphate, monosodium dihydrogen phosphate, disodium hydrogen phosphate, ammonium phosphate, diammonium hydrogen phosphate, ammonium dihydrogen phosphate, tripotassium phosphate, monopotassium dihydrogen phosphate, dipotassium hydrogen phosphate or a mixture thereof; wherein the silicate source is sodium silicate, potassium silicate, tetraethylorthosilicate, tetraethylsilicate or mixtures thereof; and wherein the amino acid is selected from acidic amino acid, neutral amino acid or a mixture thereof.
Owner:UNILEVER IP HLDG BV
Medicine injection for treating anaerobic bacterium infection and preparation method thereof
InactiveCN109431988AGood dispersionGood synergyAntibacterial agentsOrganic active ingredientsMedication injectionMedicine
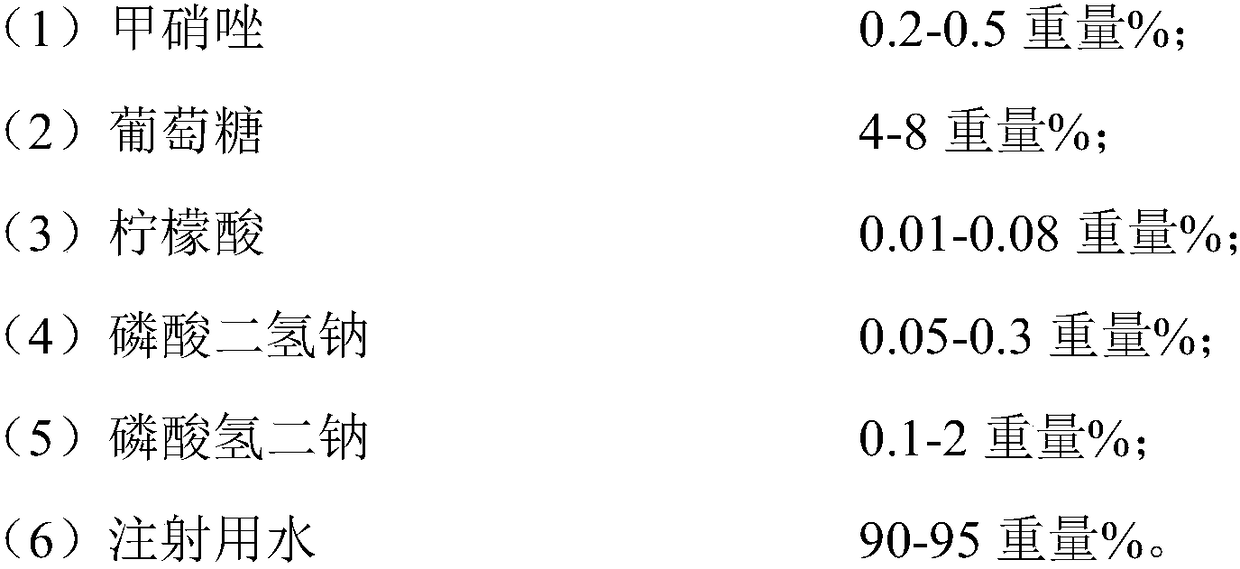
The invention relates to the field of metronidazole medicines and discloses medicine injection for treating anaerobic bacterium infection and a preparation method thereof. The injection is prepared from the following components based on the total weight of the injection: (1) 0.2 to 0.5 weight percent of metronidazole; (2) 4 to 8 weight percent of glucose; (3) 0.01 to 0.08 weight percent of citricacid; (4) 0.05 to 0.3 weight percent of sodium dihydrogen phosphate; (5) 0.1 to 2 weight percent of disodium hydrogen phosphate; (6) 90 to 95 weight percent of injection water. The medicine injectionfor treating the anaerobic bacterium infection, provided by the invention, has good stability.
Owner:江西润泽药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com