Composition and preparation method of deslanoside injection
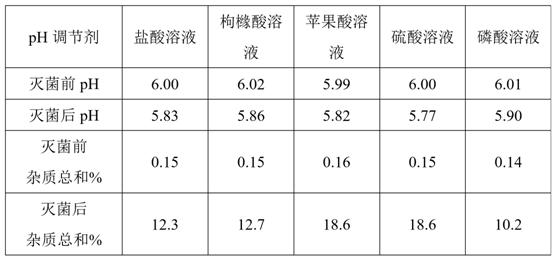
A technology for preparing deacetylated lanatosides and injections, which is applied in the direction of drug combinations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. Sterilization process, heat instability of deacetyl lanatoside, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
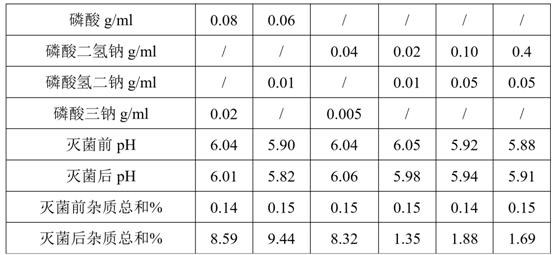
[0032]Material name Dosage / g
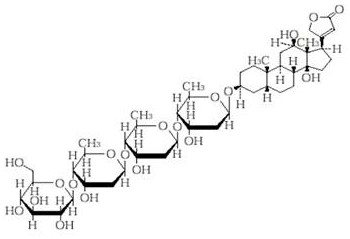
[0033]Deacetyl lanolin 0.2
[0034]Ethanol 80
[0035]Glycerin 175
[0036]Sodium dihydrogen phosphate 0.02
[0037]Disodium hydrogen phosphate 0.01
[0038]Add water for injection to 1000ml
[0039]Add glycerin, ethanol and deacetyl lanolin in the liquid preparation tank, stir until the dissolution is clear, add sodium dihydrogen phosphate and disodium hydrogen phosphate, use water for injection to sufficient, stir until the dissolution is clear, 0.22μm filter, ampoule filling Pack and fuse, autoclave at 121°C for 12 minutes.
Embodiment 2
[0041]Material name Dosage / g
[0042]Deacetyl lanolin 0.2
[0043]Ethanol 80
[0044]Glycerin 175
[0045]Sodium dihydrogen phosphate 0.8
[0046]Disodium hydrogen phosphate 0.09
[0047]Add water for injection to 1000ml
[0048]Add glycerin, ethanol and deacetyl lanolin in the liquid preparation tank, stir until the dissolution is clear, add sodium dihydrogen phosphate and disodium hydrogen phosphate, use water for injection to sufficient, stir until the dissolution is clear, 0.22μm filter, ampoule filling Pack and fuse, autoclave at 121°C for 20 minutes.
Embodiment 3
[0050]Material name Dosage / g
[0051]Deacetyl lanolin 0.2
[0052]Ethanol 80
[0053]Glycerin 175
[0054]Sodium dihydrogen phosphate 0.7
[0055]Disodium hydrogen phosphate 0.1
[0056]Add water for injection to 1000ml
[0057]Add glycerin, ethanol and deacetyl lanolin in the liquid preparation tank, stir until the dissolution is clear, add sodium dihydrogen phosphate and disodium hydrogen phosphate, use water for injection to sufficient, stir until the dissolution is clear, 0.22μm filter, ampoule filling Pack and fuse, autoclave at 121°C for 15 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com